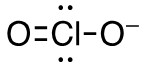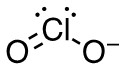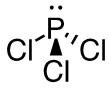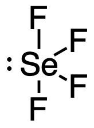Chemical Bonding — Molecular Geometry and Hybridization of Atomic Orbitals: Determine Electron Pair Geometry and Molecular Geometry
You may choose to prevent this website from aggregating and analyzing the actions you take here. Doing so will protect your privacy, but will also prevent the owner from learning from your actions and creating a better experience for you and other users.
This opt out feature requires JavaScript.
Question
What are the electron-pair geometry and the molecular geometry of each of the following molecules or ions?
- ClF5
- ClO2–
- PCl3
- SeF4
- PH2–
Show/Hide Answer
- Electron-pair geometry: octahedral
Molecular geometry: square pyramidal - Electron-pair geometry: tetrahedral
Molecular geometry: bent - Electron-pair geometry: tetrahedral
Molecular geometry: trigonal bipyramidal - Electron-pair geometry: trigonal bipyramidal
Molecular geometry: seesaw - Electron-pair geometry: tetrahedral
Molecular geometry: bent
Refer to Section 5.1: Molecular Structure and Polarity (1).
Strategy Map
Do you need a little help to get started?
Check out the strategy map.
Show/Hide Strategy Map
| Strategy Map Steps |
|---|
| 1. Create a Lewis structure for the molecule. |
2. Identify how many total electron domains are attached to the centre atom
Show/Hide HintElectron domains are all the bonding domains and lone pairs attached to the centre atom. Example: PCl3 has 4 electron domains
|
3. Identify how many of the electron domains are lone pairs. This will determine the molecules’ molecular geometry.
Show/Hide HintLone pairs will repel the other atoms and distort the molecules’ shape. Example: In PCl3, the lone pairs attached to phosphorus push the chlorine atoms downwards.
|
Solution
Do you want to see the steps to reach the answer?
Check out this solution.
Show/Hide Solution
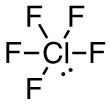
a. ClF5
- 42 valence electrons; Cl will be central atom.
- 6 electron domains (The centre chlorine atom is attached to 5 fluorines and has 1 lone pair).
Answer:
- Electron-pair geometry: octahedral
- Molecular geometry: square pyramidal
b. ClO2–
- 17 valence electrons; Cl will be central atom.
- 4 electron domains (The centre chlorine atom is attached to 2 oxygens and has 2 lone pairs).
Answer:
- Electron-pair geometry: tetrahedral
- Molecular geometry: bent
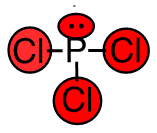
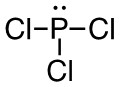
c. PCl3
- 42 valence electrons; P will be central atom.
- 4 electron domains (The centre phosphorus atom is attached to 3 chorines and has 1 lone pair).
Answer:
- Electron-pair geometry: tetrahedral
- Molecular geometry: trigonal bipyramidal
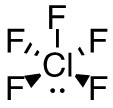
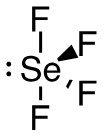
d. SeF4
- 44 valence electrons; Se will be central atom.
- 5 electron domains (The centre selenium is attached to 4 fluorines and has 1 lone pair).
Answer:
- Electron-pair geometry: trigonal bipyramidal
- Molecular geometry: seesaw
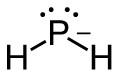
e. PH2–
- 8 valence electrons; P will be central atom
- 4 electron domains (The centre phosphorus atom is attached to 2 hydrogens and has 2 lone pairs).
Answer:
- Electron-pair geometry: tetrahedral
- Molecular geometry: bent
Guided Solution
Do you want more help?
The guided solution below will give you the reasoning for each step to get your answer, with reminders and hints.
Show/Hide Guided Solution
| Guided Solution Ideas |
|---|
This question is a theory problem where you are asked to determine both the electron-pair geometries (the name for the shape given the number of domains) and the molecular geometries (the name of the shape depending on the electron cloud distortions) for each of the given molecules.
Show/Hide Resource
|
To figure out how many electron domains are in the molecule, we must first draw the molecule with all lone pairs and bonds.
Show/Hide Think About This!We can do this easily by first creating the molecule’s Lewis structure. Example: The Lewis structure below shows that the molecule has 4 electron domains.
|
Why is the electron-pair geometry different from the molecular geometry?
Show/Hide Think About This!Recall that the electron-pair geometry is the basic shape with a certain number of electron domains. The molecular geometry is the shape the molecule displays of the bonding domains, which is due to electron pair repulsions. If there are lone pairs attached to the central atom, they influence the shape of the molecule. The molecular geometry can be the same as the electron-pair geometry if the central atom does not have any lone pairs. Example:
|
| Complete Solution |
|---|
| a. ClF5
6 electron domains (The centre chlorine atom is attached to 5 fluorines and has 1 lone pair). Lewis Structure: Molecular Geometry (with lone pairs): Answer:
|
| b. ClO2–
4 electron domains (The centre chlorine atom is attached to 2 oxygens and has 2 lone pairs). Lewis Structure:
Molecular Geometry (with lone pairs):
Answer:
|
| c. PCl3
4 electron domains (The centre phosphorus atom is attached to 3 chorines and has 1 lone pair). Lewis Structure:
Molecular Geometry (with lone pairs):
Answer:
|
| d. SeF4
5 electron domains (The centre selenium is attached to 4 fluorines and has 1 lone pair).
Molecular Geometry (with lone pairs):
Answer:
|
| e. PH2–
4 electron domains (The centre phosphorus atom is attached to 2 hydrogens and has 2 lone pairs). Lewis Structure:
Molecular Geometry (with lone pairs):
Answer:
|
Check Your Work
Electron pair geometry depends on the electron pair domains around a central atom. The molecular or species shape then describes how the bonds are arranged around the central atom.
Show/Hide Check Your Work!
Making sure that your Lewis structure is correctly drawn is very important before determining shape. Pay attention to whether the species is neutral or charged. Make sure you have counted valence electrons properly.
Does your answer make chemical sense?
Show/Hide Answer
Previously, we had been looking at molecules 2 dimensionally, as if they were flat like a paper. With molecular geometry, we begin to see that molecules have 3-dimensional shapes that moves freely. They will move into positions that require the least amount of energy. This means that the outside atoms will move as far as they can from other negative charges (i.e., other atoms and electrons). Lone-paired electrons are very repulsive and, thus, will cause even more distortion within an atom, giving it a 3-dimensional shape.
Provide feedback by taking the survey here: link to survey

PASS Attribution
- LibreTexts PASS Chemistry Book CHEM 1500 (2).
- Question 5.E.09 from LibreTexts PASS Chemistry Book CHEM 1500 (3) is used under a CC BY-NC-SA 4.0 license.
- Question 5.E.09 is a question from Section 7.E.6: 7.7: Molecular Structure and Polarity from LibreTexts Chemistry 1e (OpenSTAX) (4), which is under a CC BY 4.0 license.
- Question 7.E.6:7.7 is question 93(a,b,d,e,f) from OpenStax Chemistry (5), which is under a CC BY 4.0 license. Access for free at https://openstax.org/books/chemistry/pages/1-introduction.
Media Attributions
All figures by the authors (Brewer S. and Blackstock L.) are free to use under a CC0 license.
References
1. OpenStax. 5.1: Molecular Structure and Polarity. In CHEM 1500: Chemical Bonding and Organic Chemistry; LibreTexts, 2022. https://chem.libretexts.org/Courses/Thompson_Rivers_University/CHEM_1500%3A_Chemical_Bonding_and_Organic_Chemistry/05%3A_Chemical_Bonding_II-_Molecular_Geometry_and_Hybridization_of_Atomic_Orbitals/5.01%3A_Molecular_Structure_and_Polarity.
2. Blackstock, L.; Brewer, S.; Jensen, A. PASS Chemistry Book CHEM 1500; LibreTexts, 2023. https://chem.libretexts.org/Courses/Thompson_Rivers_University/PASS_Chemistry_Book_CHEM_1500.
3. Blackstock, L.; Brewer, S.; Jensen, A. 5.1: Question 5.E.09 PASS – Determine Electron Pair Geometry and Molecular Geometry. In PASS Chemistry Book CHEM 1500; LibreTexts, 2023. https://chem.libretexts.org/Courses/Thompson_Rivers_University/PASS_Chemistry_Book_CHEM_1500/05%3A_Chemical_Bonding_II_-_Molecular_Geometry_and_Hybridization_of_Atomic_Orbitals/5.01%3A_Question_5.E.09_PASS_-_determine_electron_pair_geometry_and_molecular_geometry.
4. OpenStax. 7.E: Chemical Bonding and Molecular Geometry (Exercises). In Chemistry 1e (OpenSTAX); LibreTexts, 2022. https://chem.libretexts.org/Bookshelves/General_Chemistry/Chemistry_1e_(OpenSTAX)/07%3A_Chemical_Bonding_and_Molecular_Geometry/7.E%3A_Chemical_Bonding_and_Molecular_Geometry_(Exercises).
5. Flowers, P.; Robinson, W. R.; Langley, R.; Theopold, K. Ch. 7 Exercises. In Chemistry; OpenStax, 2015. https://openstax.org/books/chemistry/pages/7-exercises.