Chemical Bonding — Molecular Geometry and Hybridization of Atomic Orbitals: Using Molecular Orbital Theory and Bond Order to Determine Ion Charge
You may choose to prevent this website from aggregating and analyzing the actions you take here. Doing so will protect your privacy, but will also prevent the owner from learning from your actions and creating a better experience for you and other users.
This opt out feature requires JavaScript.
Question
What charge would be needed on F2 to generate an ion with a bond order of 2?
Show/Hide Answer
The charge would be 2+.
Refer to Section 5.5: Molecular Orbital Theory (1).
Strategy Map
Do you need a little help to get started?
Check out the strategy map.
Show/Hide Strategy Map
| Strategy Map Steps |
|---|
1. Fill out a molecular orbital energy diagram.
Show/Hide HintRecall molecular orbital energy diagrams. Refer to Section 5.5.3: Bonding Diatomic Molecules (1). |
2. Calculate the bond order of F2.
Show/Hide HintRecall the bond order formula. Refer to Section 5.5.3: Bonding Diatomic Molecules (1). |
| 3. Calculate the number of electrons it would require for a bond order of 2. |
| 4. Identify the electron difference and if you would have to add or subtract electrons to F2 to fix it. |
Solution
Do you want to see the steps to reach the answer?
Check out this solution.
Show/Hide Solution
Bond order (BO) formula:
[latex]\begin{gathered} \mathrm{BO}=\frac{\text{(Number of bonding electrons)}-\text{(Number of antibonding electrons)}}{2} \end{gathered}[/latex]
The typical bond order for F2:
[latex]\begin{aligned} \mathrm{BO}&=\frac{\text{Bonding electrons}-\text{Antibonding electrons}}{2}\\ \mathrm{BO}&=\frac{8-6}{2}\\ \mathrm{BO}&=1 \end{aligned}[/latex]
Electrons required for bond order of 2:
[latex]\begin{aligned} \mathrm{BO}&=\frac{\text{Bonding electrons }-\text{Antibonding electrons}}{2}\\ 2&=\frac{\mathrm{X}}{2}\\ \mathrm{X}&=4 \end{aligned}[/latex]
Answer: Two electrons would have to be removed, therefore the charge on the F2 molecule would be 2+.
Guided Solution
Do you want more help?
The guided solution below will give you the reasoning for each step to get your answer, with reminders and hints.
Show/Hide Guided Solution
| Guided Solution Ideas |
|---|
This question is both a theory and calculation type problem where you will use the bond order equation to find the charge a polyatomic ion would require with a bond order of 2.
Show/Hide Resource
|
| Try filling out a molecular orbital energy diagram for F2:
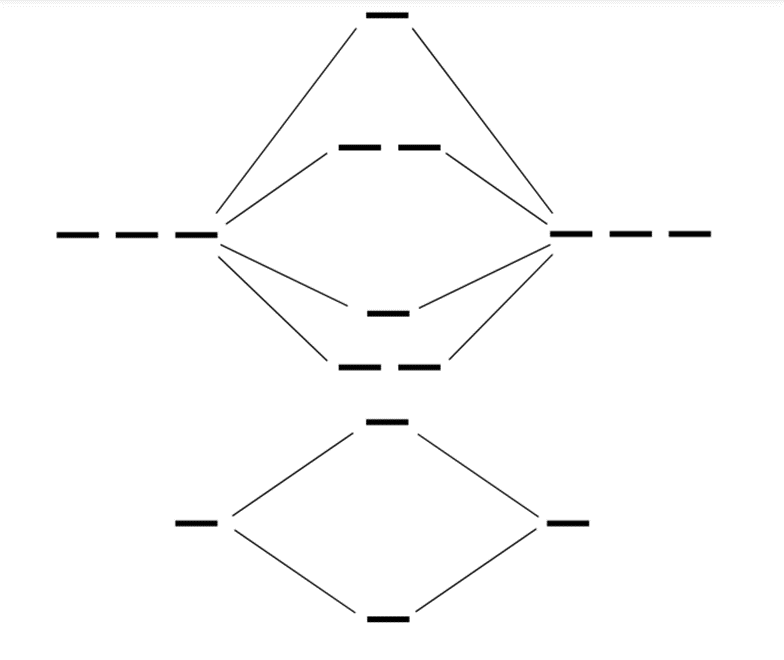
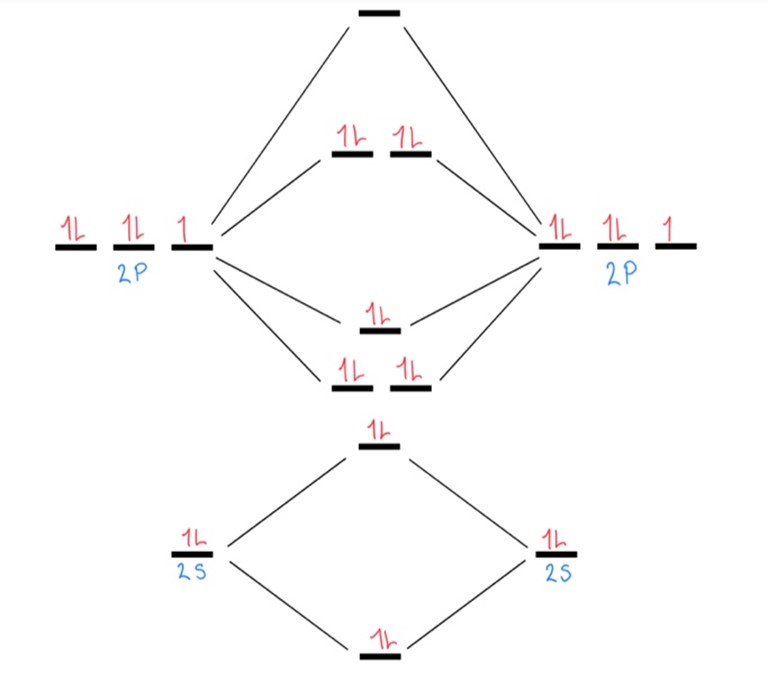
This shows how many bonding and antibonding electrons are in this polyatomic ion, which you will use for your calculation. Show/Hide Think About This!Filled out diagram:
|
Recall the bond order (BO) equation.
Show/Hide Don’t Forget![latex]\begin{gathered} \mathrm{BO}= \frac{\text{(Number of bonding electrons)}-\text{(Number of antibonding electrons)}}{2} \end{gathered}[/latex] |
Since we are adding a charge to the polyatomic ion, we know that we are manipulating the number of electrons it has. This means you will have to identify the difference in electrons for the polyatomic ions normal bond order and if it were to have a bond order of 2.
Show/Hide Think About This!Use the bond order equation to calculate the number of electrons it would require having a bond order of 2: [latex]\begin{equation} 2=\frac{\mathrm{X}}{2} \end{equation}[/latex] Subtract the number you get for “x” from the number of electrons F2 would usually have. |
Will the charge be positive or negative?
Show/Hide Think About This!Recall that when you add electrons to an atom, it gains a negative charge, and when you remove electrons, its gains a positive charge. |
| Complete Solution |
|---|
| Bond order (BO) formula:
[latex]\begin{gathered} \mathrm{BO}=\\ \frac{\text{(Number of bonding electrons)}-\text{(Number of antibonding electrons)}}{2} \end{gathered}[/latex] |
| The typical bond order for F2:
Molecular orbital diagram:
There are 8 bonding electrons and 6 antibonding electrons in F2: [latex]\begin{aligned} \mathrm{BO}&=\frac{\text{Bonding electrons}-\text{Antibonding electrons}}{2} \\ \mathrm{BO}&=\frac{8-6}{2}\\ \mathrm{BO}&=1 \end{aligned}[/latex] The bond order for a ground state F2 polyatomic ion would be 4. |
| Electrons required for bond order of 2:
Since we know we want the bond order to be 2, but we will not know the number of electrons that would be, we can use the same formula to isolate and solve for the number of electrons. [latex]\begin{aligned} \mathrm{BO}&=\frac{\text{Bonding electrons}-\text{Antibonding electrons }}{2}\\ 2&=\frac{\mathrm{x}}{2}\\ \mathrm{X}&=4 \end{aligned}[/latex] If we remove 2 of the antibonding electrons from F2, its new bond order would be 2. [latex]\begin{gathered} \mathrm{BO}=\frac{8-4}{2}=\frac{4}{2}=2 \end{gathered}[/latex] Answer: Since 2 electrons are being removed, F2 would gain a positive charge of 2 making the polyatomic ion F22+. |
Check Your Work
For a bond order of 2, 2 electrons have been removed from F2, leaving it with a charge of positive 2.
Does your answer make chemical sense?
Show/Hide Check Your Work!
The bond order is the number of bonds in a molecule or polyatomic ion. The higher the bond order, the stronger the bond. It makes sense for the bond order to increase if two electrons are removed from F2, the single bonded atom would have to become double bonded to fulfill the octet rule. A double bond is much stronger than a single bond, this can be mathematically seen when looking at the table of bond energies, it requires significantly more energy to break a double bond than a single bond.
Provide feedback by taking the survey here: link to survey

PASS Attribution
- LibreTexts PASS Chemistry Book CHEM 1500 (2).
- Question 5.E.78 from LibreTexts PASS Chemistry Book CHEM 1500 (3) is used under a CC BY-NC-SA 4.0 license.
- Question 5.E.78 is question 78 (8.E.2: Chemistry End of Chapter Exercises) from LibreTexts Chemistry 1e (OpenSTAX) (4), which is under a CC BY 4.0 license.
- Question 78 is question 47 from OpenStax Chemistry (5), which is under a CC BY 4.0 license. Access for free at https://openstax.org/books/chemistry/pages/1-introduction.
Media Attribution
All figures are by Ashlynn Jensen, from LibreTexts PASS Chemistry Book CHEM 1500 (3) by Blackstock et al., and are used/updated under a CC BY-NC 4.0 license.
References
1. OpenStax. 5.5: Molecular Orbital Theory. In CHEM 1500: Chemical Bonding and Organic Chemistry; LibreTexts, 2022. https://chem.libretexts.org/Courses/Thompson_Rivers_University/CHEM_1500%3A_Chemical_Bonding_and_Organic_Chemistry/05%3A_Chemical_Bonding_II-_Molecular_Geometry_and_Hybridization_of_Atomic_Orbitals/5.05%3A_Molecular_Orbital_Theory.
2. Blackstock, L.; Brewer, S.; Jensen, A. PASS Chemistry Book CHEM 1500; LibreTexts, 2023. https://chem.libretexts.org/Courses/Thompson_Rivers_University/PASS_Chemistry_Book_CHEM_1500.
3. Blackstock, L.; Brewer, S.; Jensen, A. 5.4: Question 5.E.78 PASS – Using Molecular Orbital Theory and Bond Order to Determine Ion Charge. In PASS Chemistry Book CHEM 1500; LibreTexts, 2023. https://chem.libretexts.org/Courses/Thompson_Rivers_University/PASS_Chemistry_Book_CHEM_1500/05%3A_Chemical_Bonding_II_-_Molecular_Geometry_and_Hybridization_of_Atomic_Orbitals/5.04%3A_Question_5.E.78_PASS_-_using_molecular_orbital_theory_and_bond_order_to_determine_ion_charge.
4. OpenStax. 8.E: Advanced Theories of Covalent Bonding (Exercises). In Chemistry 1e (OpenSTAX); LibreTexts, 2022. https://chem.libretexts.org/Bookshelves/General_Chemistry/Chemistry_1e_(OpenSTAX)/08%3A_Advanced_Theories_of_Covalent_Bonding/8.E%3A_Advance_Theories_of_Covalent_Bonding_(Exercises).
5. Flowers, P.; Robinson, W. R.; Langley, R.; Theopold, K. Ch. 8 Exercises. In Chemistry; OpenStax, 2015. https://openstax.org/books/chemistry/pages/8-exercises.