Organic Chemistry — Bonding and Structure: Naming Cycloalkane Structures
You may choose to prevent this website from aggregating and analyzing the actions you take here. Doing so will protect your privacy, but will also prevent the owner from learning from your actions and creating a better experience for you and other users.
This opt out feature requires JavaScript.
Question
Give the IUPAC names for the following cycloalkane structures:
-
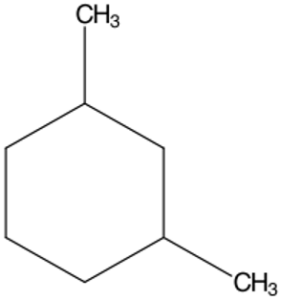
Structure A -
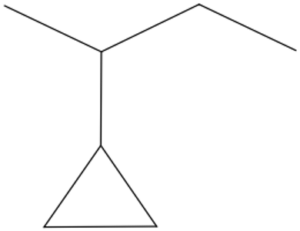
Structure B -
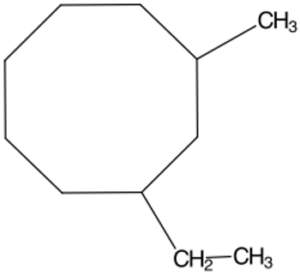
Structure C -
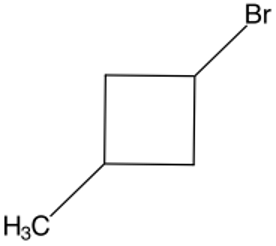
Structure D -
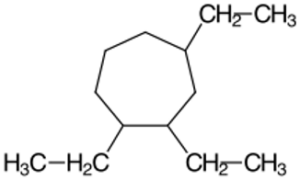
Structure E -
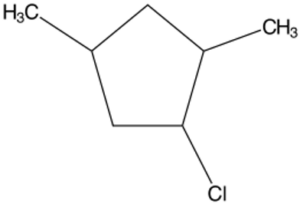
Structure F
Show/Hide Answer
- 1,3-Dimethylcyclohexane
- 2-Cyclopropylbutane
- 1-Ethyl-3-methylcyclooctane
- 1-Bromo-3-methylcyclobutane
- 1,2,4-Triethylcycloheptane
- 1-Chloro-2,4-dimethylcyclopentane
Refer to Section 7.5.2: Naming Cyclohexanes (1).
Strategy Map
Do you need a little help to get started?
Check out the strategy map.
Show/Hide Strategy Map
| Strategy Map Steps |
|---|
| 1. Identify the longest chain (making up the ring or attached to it). |
| 2. Count the number of carbons in the ring and along the carbon chains. |
3. Number the connecting chains/rings in alphabetical order.
Show/Hide HintEnsure that you number each connecting chain going along the path that allows for the lowest numbers (either left or right). |
Solution
Do you want to see the steps to reach the answer?
Check out this solution.
Show/Hide Solution
b. Structure B
- The longest carbon chain is in the form of a 4-carbon long chain.
- Attached to the second carbon of the chain is a 3-carbon long ring.
Answer: 2-Cyclopropylbutane
c. Structure C
- The longest chain is an 8-carbon long ring.
- Attached to the first carbon on the ring is a 2-carbon long chain.
- Attached to the third carbon in the ring is a 1-carbon long chain.
Answer: 1-Ethyl-3-methylcyclooctane
d. Structure D
- The longest chain is a 4-carbon long ring.
- Attached to the first carbon on the ring is a bromine.
- Attached to the third carbon on the ring is a 1-carbon long chain.
Answer: 1-Bromo-3-methylcyclobutane
e. Structure E
- The longest chain is a 5-carbon long ring.
- Attached to the first carbon on the ring is a chlorine atom.
- Two 1-carbon long chains are attached on carbons 2 and 4 of the ring.
Answer: 1-Chloro-2,4-dimethylcyclopentane
Guided Solution
Do you want more help?
The guided solution below will give you the reasoning for each step to get your answer, with reminders and hints.
Show/Hide Guided Solution
| Guided Solution Ideas |
|---|
This question is a theory type problem where you will need to be able to name different types of cycloalkanes.
Show/Hide Resource
|
Find which chain is the longest. This can either be inside of the ring or attached to the outside, but you cannot count inside to outside or vice versa.
Show/Hide Don’t Forget!This part will go at the end of the name. Show/Hide Don’t Forget!Recall the names for your carbon chains.
If it is a ring, just put “cyclo” in front! |
Identify your connecting chain(s).
Show/Hide Don’t Forget!These will be named and numbered based on their order in the alphabet. Show/Hide Don’t Forget!If you have 2 of the same chains attached to separate carbons on the main chain, they will be listed in order of number (e.g., -1, 3-methyl). |
| Complete Solution |
|---|
b. Structure B
Answer: 2-Cyclopropylbutane |
c. Structure C
Answer: 1-Ethyl-3-methylcyclooctane |
d. Structure D
Answer: 1-Bromo-3-methylcyclobutane |
e. Structure E
Answer: 1-Chloro-2,4-dimethylcyclopentane |
Check Your Work
Summary of what we would expect based on the related chemistry theory
Show/Hide Check Your Work!
To check if your answer is correct, look back to ensure you followed all of the rules and guidelines. If you accurately named the compound, you should be able to redraw it using your name. The compound you draw should match the original molecule. If it does not, you know you made an error in your naming process.
Does your answer make chemical sense?
Show/Hide Answer
To answer this question, you have followed the IUPAC rules for naming cycloalkanes. The key is to make sure your longest chain is the parent part of the name. The longest chain may be a straight chain alkane with the cylcoalkane as a substituent. If you focus on identifying the longest chain first and number your carbons to end up with the lowest possible substituent numbers, then you have followed the correct approach.
Show/Hide Resource!
Refer to Section 7.5.2: Naming Cyclohexanes (1).
Provide feedback by taking the survey here: link to survey

PASS Attribution
- LibreTexts PASS Chemistry Book CHEM 1500 (2).
- Question 7.5.2.1 from LibreTexts PASS Chemistry Book CHEM 1500 (3) is used under a CC BY-NC 4.0 license.
- Question 7.5.2.1 is question 7.5.2.1 from LibreTexts CHEM 1500: Chemical Bonding and Organic Chemistry (1) is used under a CC BY-SA 4.0 license.
- Question 7.5.2.1 is question 4.1.2 from LibreTexts Organic Chemistry (Morsch et al.) (4), which is under a CC BY-SA 4.0 license.
Media Attributions
All figures are by Ashlynn Jensen, from LibreTexts PASS Chemistry Book CHEM 1500 (3) by Blackstock et al., and are used/updated under a CC BY-NC 4.0 license.
References
1. Clark, J.; Farmer, S.; Kennepohl, D.; Sharrett, Z.; Morsch, L.; Cunningham, K.; Soderberg, T.; Zin, P.; Matthews, K. 7.5.2 Naming Cycloalkanes. In CHEM 1500: Chemical Bonding and Organic Chemistry; LibreTexts, 2023. https://chem.libretexts.org/Courses/Thompson_Rivers_University/CHEM_1500%3A_Chemical_Bonding_and_Organic_Chemistry/07%3A_Organic_Chemistry_I_-_Bonding_and_Structure/7.05%3A_Naming_of_Alkanes_and_Cycloalkanes/7.5.02%3A_Naming_Cycloalkanes
2. Blackstock, L.; Brewer, S.; Jensen, A. PASS Chemistry Book CHEM 1500; LibreTexts, 2023. https://chem.libretexts.org/Courses/Thompson_Rivers_University/PASS_Chemistry_Book_CHEM_1500.
3. Blackstock, L.; Brewer, S.; Jensen, A. 7.4: Question 7.5.2.1 PASS – Naming Cycloalkane Structures. In PASS Chemistry Book CHEM 1500; LibreTexts, 2023. https://chem.libretexts.org/Courses/Thompson_Rivers_University/PASS_Chemistry_Book_CHEM_1500/07%3A_Organic_Chemistry_I_-_Bonding_and_Structure/7.04%3A_Question_7.5.2.1_PASS_-_naming_cycloalkane_structures.
4. Clark, J.; Farmer, S.; Kennepohl, D.; Sharrett, Z.; Morsch, L.; Cunningham, K.; Soderberg, T.; Zin, P.; Matthews, K. 4.1: Naming Cycloalkanes. In Organic Chemistry (Morsch et al.); LibreTexts, 2024. https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Organic_Chemistry_(Morsch_et_al.)/04%3A_Organic_Compounds_-_Cycloalkanes_and_their_Stereochemistry/4.01%3A_Naming_Cycloalkanes.

