Organic Chemistry – Stereochemistry: Enantiomers, Diastereomers, Identical
You may choose to prevent this website from aggregating and analyzing the actions you take here. Doing so will protect your privacy, but will also prevent the owner from learning from your actions and creating a better experience for you and other users.
This opt out feature requires JavaScript.
Question
For the following compounds, identify whether they are enantiomers, diastereomers, or the same compounds.
-
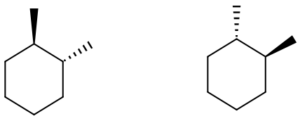
Compounds A, Enantiomers Diastereomers Identical 1 -
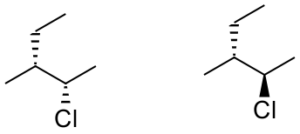
Compounds B, Enantiomers Diastereomers Identical 2 -
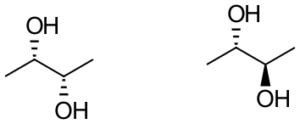
Compounds C, Enantiomers Diastereomers Identical 3 -

Compounds D, Enantiomers Diastereomers Identical 4
Show/Hide Answer
- Enantiomers
- Diastereomers
- Diastereomers
- Same compound
Refer to Section 8.1: Types of Isomers (1).
Strategy Map
Do you need a little help to get started?
Check out the strategy map.
Show/Hide Strategy Map
| Strategy Map Steps |
|---|
1. Use your knowledge on compound sketches to “flip” 1 of the compounds.
Show/Hide HintWhen we say “flip” a compound, we are referring to rotating its orientation. This causes dashed-back atoms to become wedged forward and vice versa. From this, we can see if the 2 compounds are the same and how they differ. |
| 2. Identify if both compounds have the same shape or are optical isomers. |
| 3. If they are optical isomers, identify if they are mirror images of each other. |
Solution
Do you want to see the steps to reach the answer?
Check out this solution.
Show/Hide Solution
a. Compounds A
These compounds are mirror image optical isomers.
Answer: Enantiomers
b. Compounds B
These compounds are non-mirror image optical isomers.
Answer: Diastereomers
c. Compounds C
These compounds are non-mirror image optical isomers.
Answer: Diastereomers
d. Compounds D
These compounds have the same chemical formula and connectivity.
Answer: Same compound
Guided Solution
Do you want more help?
The guided solution below will give you the reasoning for each step to get your answer, with reminders and hints.
Show/Hide Guided Solution
| Guided Solution Ideas |
|---|
This question is a theory type problem that requires you to identify if 2 compounds are optical isomers and what type.
Show/Hide Resource
|
Recall what these terms mean:
|
Recall how you know the 2 compounds are the same.
Show/Hide Think About This!If 2 compounds are the same, they will have the same formula and connectivity. When you “flip” the compound, you should be able to superimpose it on the other. |
Recall how to “flip” the compound.
Show/Hide Think About This!When we say “flipping” compounds, we refer to rotating its orientation. This causes dashed-back atoms to become wedged forward and vice versa. From this, we can see if the 2 compounds are the same and how they differ. |
| Complete Solution |
|---|
| a. Compounds A
These compounds are mirror image optical isomers. These compounds are nonsuperimposable. The 2 methane groups have opposing orientations. On one compound, the first methane group is wedged and the second is dashed; on the other compound the first methane group is dashed and the second is wedged. Answer: Enantiomers |
| b. Compounds B
These compounds are non-mirror image optical isomers. These compounds are nonsuperimposable. On one compound, the chlorine is dashed back; on the other, the chlorine is wedged forwards. All other connectivity is the same. Answer: Diastereomers |
| c. Compounds C
These compounds are non-mirror image optical isomers. These compounds are nonsuperimposable. On one compound, the hydroxyl group is dashed back; on the other, the hydroxyl group is wedged forwards. All other connectivity is the same. Answer: Diastereomers |
| d. Compounds D
These compounds have the same chemical formula and connectivity. They are superimposable. Answer: Same compound |
Check Your Work
Summary of what we would expect based on the related chemistry theory
Does your answer make chemical sense?
Show/Hide Check Your Work!
It is important to be able to identify the different types of isomers. The easiest way to check optical isomers is to imagine yourself trying to superimpose them (or doing so with a molecular model kit). This will allow you to see which bonds are different. Ensure that all your answers follow the rules.
Refer to Figure 8.1.1 Different Types of Isomers (1).
Provide feedback by taking the survey here: link to survey

PASS Attribution
- LibreTexts PASS Chemistry Book CHEM 1500 (2).
- Question 8.4.E.2 from LibreTexts PASS Chemistry Book CHEM 1500 (3) is used under a CC BY-NC 4.0 license.
- Question 8.4.E.2 is question 6-11 from LibreTexts SCC: Chem 420 – Organic Chemistry I (4), which is used under a CC BY-NC-SA 4.0 license.
Media Attributions
All figures are by Ashlynn Jensen, from LibreTexts PASS Chemistry Book CHEM 1500 (2) by Blackstock et al., and are used/updated under a CC BY-NC 4.0 license.
References
1. Farmer, S.; Kennepohl, D.; Sharrett, Z.; Reusch, W. 8.1: Types of Isomers. In CHEM 1500: Chemical Bonding and Organic Chemistry; LibreTexts, 2022. https://chem.libretexts.org/Courses/Thompson_Rivers_University/CHEM_1500%3A_Chemical_Bonding_and_Organic_Chemistry/08%3A_Organic_Chemistry_II_-_Stereochemistry/8.01%3A_Types_of_Isomers.
2. Blackstock, L.; Brewer, S.; Jensen, A. PASS Chemistry Book CHEM 1500; LibreTexts, 2023. https://chem.libretexts.org/Courses/Thompson_Rivers_University/PASS_Chemistry_Book_CHEM_1500.
3. Blackstock, L.; Brewer, S.; Jensen, A. 8.4 Question 8.4.E.2 PASS – Enantiomers, Diastereomers, Identical. In PASS Chemistry Book CHEM 1500; LibreTexts, 2023. https://chem.libretexts.org/Courses/Thompson_Rivers_University/PASS_Chemistry_Book_CHEM_1500/08%3A_Organic_Chemistry_II_-_Stereochemistry/8.Re%3A_Question_8.Re-6.11(other)_PASS_-_enantiomers_diastereomers_identical.
4. Sacramento City College. 6.14: Additional Exercises. In SCC: Chem 420 – Organic Chemistry I; LibreTexts, 2019. https://chem.libretexts.org/Courses/Sacramento_City_College/SCC%3A_Chem_420_-_Organic_Chemistry_I/06%3A_Stereochemistry_at_Tetrahedral_Centers/6.14%3A_Additional_Exercises.

