Thermochemistry: Using Enthalpy of Formation to Calculate ΔH
You may choose to prevent this website from aggregating and analyzing the actions you take here. Doing so will protect your privacy, but will also prevent the owner from learning from your actions and creating a better experience for you and other users.
This opt out feature requires JavaScript.
Question
In the mid-1700s, a method was devised for preparing chlorine gas from the following reaction:
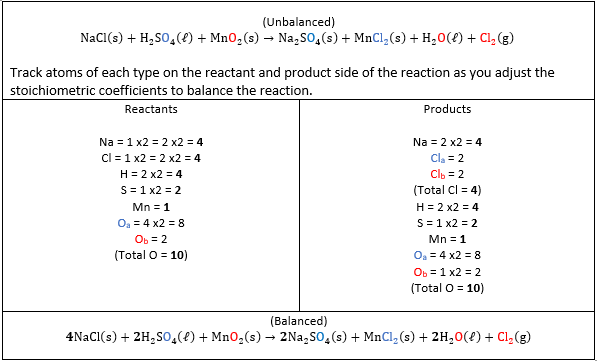
[latex]\begin{equation} \begin{gathered} \mathrm{NaCl}\,\mathrm{(s)}+\mathrm{H}_2 \mathrm{SO}_4(\mathrm{l})+\mathrm{MnO}_2(\mathrm{s})\rightarrow\mathrm{Na}_2\mathrm{SO}_4(\mathrm{s})\mathrm{MnCl}_2(\mathrm{s})+\mathrm{H}_2\mathrm{O}(\ell)+\mathrm{Cl}_2(\mathrm{g}) \end{gathered} \end{equation}[/latex]
Use the data in table T1: Standard Thermodynamic Quantities (1) and the given data below to calculate ΔH for this reaction. Is the reaction exothermic or endothermic?
[latex]\begin{equation} \begin{gathered} \Delta{\mathrm{H}^{\circ}}_\mathrm{f}\;\mathrm{H}_2\mathrm{SO}_4(\ell)=-814.0\mathrm{~kJ}/\mathrm{mol} \end{gathered} \end{equation}[/latex]
Show/Hide Answer
−34 kJ/mol; exothermic
Refer to Section 3.7: Enthalpies of Formation (2).
Strategy Map
Do you need a little help to get started?
Check out the strategy map.
Show/Hide Strategy Map
| Strategy Map Steps |
|---|
1. Make sure the reaction is balanced.
Show/Hide ResourceSee Section 1.4.2: Writing and Balancing Chemical Equations (3). |
| 2. Identify the stoichiometric coefficient of each compound. |
| 3. Use the provided table and identify the enthalpy of formation for each compound. |
4. Recall the equation to find the total enthalpy of formation for a reaction.
Show/Hide Don’t Forget!Remember that this is the sum of all enthalpies of formation where reactants are broken (which requires energy – ) and products are formed (which releases energy + ). |
| 5. Plug into the equation and solve for the total enthalpy of formation. |
Solution
Do you want to see the steps to reach the answer?
Check out this solution.
Show/Hide Solution
[latex]\begin{equation} \begin{aligned} \Delta\mathrm{H}^{\circ}{ }_{\mathrm{rxn}}&=\sum\mathrm{m}\Delta\mathrm{H}^{\circ}{ }_{\mathrm{f}}(\text{products})\sum \mathrm{n}\Delta\mathrm{H}^{\circ}{ }_{\mathrm{f}}(\text{reactants})\\ \Delta\mathrm{H}^{\circ}{ }_{\mathrm{rxn}}&=\left(\mathrm{m}\Delta\mathrm{H}^{\circ}{ }_{\mathrm{f}}\left(\mathrm{Na}_2\mathrm{SO}_4\right)+\mathrm{m}\Delta\mathrm{H}^{\circ}{ }_{\mathrm{f}}\left(\mathrm{MnCl}_2\right)+\mathrm{m}\Delta\mathrm{H}^{\circ}{ }_{\mathrm{f}}\left(\mathrm{H}_2\mathrm{O}\right)\right)-\\ &\left(\mathrm{n}\Delta\mathrm{H}^{\circ}{ }_{\mathrm{f}}(\mathrm{NaCl})+\mathrm{n}\Delta\mathrm{H}^{\circ}\mathrm{f}\left(\mathrm{H}_2\mathrm{SO}_4\right)+\mathrm{n}\Delta\mathrm{H}_{\mathrm{f}}^{\circ}{}\left(\mathrm{MnO}_2\right)\right)\\ \Delta\mathrm{H}^{\circ}{ }_{\mathrm{rxn}}&=((2(-1387.1\mathrm{~kJ}/\mathrm{mol}))+(-481.3\mathrm{~kJ}/\mathrm{mol})+\\ &(2(-285.8\mathrm{~kJ}/\mathrm{mol})))-((4(-411.2\mathrm{~kJ}/\mathrm{mol}))+\\&(2(-814.0\mathrm{~kJ}))+(-520.0\mathrm{~kJ/mol}))\\\\ \Delta\mathrm{H}_{\mathrm{rxn}}^{\circ}&=(-3827.1\mathrm{~kJ/mol})-(-3792.8\mathrm{~kJ/mol})\\ &=-34.3\mathrm{~kJ/mol}=-34\mathrm{~kJ/mol} \end{aligned} \end{equation}[/latex]
Answer is negative, so the reaction is exothermic.
Answer: −34 kJ/mol; exothermic
Guided Solution
Do you want more help?
The guided solution below will give you the reasoning for each step to get your answer, with reminders and hints.
Show/Hide Guided Solution
| Guided Solution Ideas |
|---|
This question is a calculation type problem where you must use your knowledge on how each individual enthalpy of formation will add together to find the total enthalpy of a reaction.
Show/Hide Resource
|
Recall the equation for the total enthalpy of formation. Remember that the total enthalpy of formation will be the sum of all individual enthalpy of formation values.
Show/Hide Don’t Forget![latex]\begin{gathered} \Delta\mathrm{H}^{\circ}{ }_{\mathrm{rxn}}=\sum\mathrm{m}\Delta\mathrm{H}^{\circ}{ }_{\mathrm{f}}(\text {products})-\sum\mathrm{n}\Delta\mathrm{H}^{\circ}{ }_{\mathrm{f}}(\text {reactants}) \end{gathered}[/latex] (Where m and n are your stoichiometric coefficients) |
| Balance the reaction.
Recall that for a reaction to be properly balanced, there must be the same number of each element type on both the reactant and product side. In this problem, the reaction is not balanced, and you must do that as one of your steps. Stoichiometric coefficients Recall that in your formula ‘m’ and ‘n’ represent the stoichiometric coefficients. [latex]\begin{gathered} \Delta\mathrm{H}^{\circ}{ }_{\mathrm{rxn}}=\sum\mathrm{m}\Delta\mathrm{H}^{\circ}{ }_{\mathrm{f}}(\text {products})-\sum\mathrm{n}\Delta\mathrm{H}^{\circ}{ }_{\mathrm{f}}(\text{reactants}) \end{gathered}[/latex] Each individual enthalpy of formation value will be multiplied by its corresponding stoichiometric coefficient for that compound. Show/Hide Think About This!
|
Use the provided table T1: Standard Thermodynamic Quantities (1) to find all of the enthalpy of formation values. They will be in the column titled “ΔH°f (kJ/mol).”
Show/Hide Think About This!Reactants:
Products:
|
How does your answer determine if the reaction is exothermic or endothermic?
Show/Hide Don’t Forget!Recall that an endothermic reaction will require an input of heat (meaning the total enthalpy will be positive) and an exothermic reaction will release heat (meaning the total enthalpy will be negative). |
| Complete Solution |
|---|
| Balanced Equation:
[latex]\begin{equation} \begin{gathered} 4\mathrm{NaCl}\,\mathrm{(s)}+2\mathrm{H}_2\mathrm{SO}_4(\ell)+\mathrm{MnO}_2(\mathrm{s})\rightarrow2\mathrm{Na}_2\mathrm{SO}_4(\mathrm{s})\mathrm{MnCl}_2(\mathrm{s})+2\mathrm{H}_2\mathrm{O}(\ell)+\mathrm{Cl}_2(\mathrm{g}) \end{gathered} \end{equation}[/latex] Recall the equation that gives the total enthalpy of a reaction: [latex]\begin{equation} \begin{gathered} \Delta\mathrm{H}^{\circ}{ }_{\mathrm{rxn}}=\sum\mathrm{m}\Delta\mathrm{H}^{\circ}{ }_{\mathrm{f}}(\text { products })-\sum\mathrm{n}\Delta\mathrm{H}^{\circ}{ }_{\mathrm{f}}(\text {reactants}) \end{gathered} \end{equation}[/latex] |
| Identify which compounds are reactants and which are products:
[latex]\begin{equation} \begin{aligned}\Delta\mathrm{H}^{\circ}{ }_{\mathrm{rxn}}=\left(\mathrm{m}\Delta\mathrm{H}^{\circ}{ }_{\mathrm{f}}\left(\mathrm{Na}_2\mathrm{SO}_4\right)+\mathrm{m}\Delta\mathrm{H}^{\circ}{ }_{\mathrm{f}}\left(\mathrm{MnCl}_2\right)+\mathrm{m}\Delta\mathrm{H}^{\circ}{ }_{\mathrm{f}}\left(\mathrm{H}_2\mathrm{O}\right)\right)-\left(\mathrm{n}\Delta\mathrm{H}^{\circ}{ }_{\mathrm{f}}(\mathrm{NaCl})+\right.\left.\mathrm{n}\Delta\mathrm{H}^{\circ}{ }_{\mathrm{f}}\left(\mathrm{H}_2\mathrm{SO}_4\right)+\mathrm{n}\Delta\mathrm{H}^{\circ}{ }_{\mathrm{f}}\left(\mathrm{MnO}_2\right)\right) \end{aligned} \end{equation}[/latex] Plug in the stoichiometric coefficients and enthalpy of formation value that corresponds to each compound: [latex]\begin{equation} \begin{aligned} \Delta\mathrm{H}^{\circ}{ }_{\mathrm{rxn}}=((2(-1387.1\mathrm{~kJ/mol}))+(-481.3\mathrm{~kJ/mol})+(2(-285.8\mathrm{~kJ/mol})))-((4(-411.2\mathrm{~kJ/mol}))+(2(-814.0\mathrm{~kJ/mol}))+(-520.0\mathrm{~kJ/mol})) \end{aligned} \end{equation}[/latex] |
| Solve for the total enthalpy change:
[latex]\begin{equation} \begin{aligned} \Delta\mathrm{H}^{\circ}{ }_{\mathrm{rxn}}&=(-3827.1\mathrm{~kJ/mol})-(-3792.8\mathrm{~kJ/mol})\\ &=-34.3\mathrm{~kJ/mol}=-34\mathrm{~kJ/mol} \end{aligned} \end{equation}[/latex] Answer is negative, so the reaction is exothermic. This is because exothermic reactions will release more energy than they require to occur. Answer: −34 kJ/mol; exothermic |
Check Your Work
Verify your set up of the equation before you plugged in the data. Make sure that you kept the signs associated with each of the data values, and that you multiplied ΔH°f values by the appropriate stoichiometric coefficients.
Show/Hide Check Your Work!
Writing out your entire expression with all terms to calculate ΔH is good practice to avoid errors.
Watch out for math errors!
Does your answer make chemical sense?
Show/Hide Answer
The standard enthalpy of reaction (ΔHrxn) can be calculated from the sum of the standard enthalpies of formation of the products (each multiplied by its stoichiometric coefficient) minus the sum of the standard enthalpies of formation of the reactants (each multiplied by its stoichiometric coefficient)—which gives us the “products minus reactants” equation.
When using standard enthalpies of formation to calculate ΔH of a reaction, we are treating the reaction as though it is going from the reactants to the elements and then to the products. This is an application of Hess’s Law. Since enthalpy of formation is the standard enthalpy change to form 1 mole of a chemical from its elements in their standard states, we are forming products and breaking apart reactants, which gives us:
[latex]\begin{gathered} \Delta\mathrm{H}^{\circ}{ }_{\mathrm{rxn}}=\sum\mathrm{m}\Delta\mathrm{H}^{\circ}{ }_{\mathrm{f}}(\text {products})-\sum\mathrm{n}\Delta\mathrm{H}^{\circ}{ }_{\mathrm{f}}(\text {reactants}) \end{gathered}[/latex]
(Where m and n are your stoichiometry factors)
In this case, we get a negative value for ΔHrxn, which tells us the reaction is exothermic.
Show/Hide Resource.
Refer to Section 3.7: Enthalpies of Formation (2).
Provide feedback by taking the survey here: link to survey

PASS Attribution
- LibreTexts TRU: Fundamentals and Principles of Chemistry (CHEM 1510 and CHEM 1520) (4)
- Question 3.E.28 from LibreTexts TRU: Fundamentals and Principles of Chemistry (CHEM 1510 and CHEM 1520) (5) is used under a CC BY-NC-SA 4.0 license.
- Question 3.E.28 was adapted from question 9 [in the Numerical Problems section] from General Chemistry: An Atoms First Approach (6), which is under a CC BY-NC-SA 3.0 license.
Media Attributions
All figures by the authors (Brewer S. and Blackstock L.) are free to use under a CC0 license.
References
1. LibreTexts. Thermodynamics Tables T1: Standard Thermodynamic Quantities. In Reference Tables; LibreTexts, 2022. https://chem.libretexts.org/Ancillary_Materials/Reference/Reference_Tables/Thermodynamics_Tables/T1%3A_Standard_Thermodynamic_Quantities.
2. Thompson Rivers University. 3.7: Enthalpies of Formation. In TRU: Fundamentals and Principles of Chemistry (CHEM 1510 and CHEM 1520); LibreTexts, 2022. https://chem.libretexts.org/Courses/Thompson_Rivers_University/TRU%3A_Fundamentals_and_Principles_of_Chemistry_(CHEM_1510_and_CHEM_1520)/03%3A_Thermochemistry/3.07%3A_Enthalpies_of_Formation.
3. OpenStax. 1.4.2: Writing and Balancing Chemical Equations. In TRU: Fundamentals and Principles of Chemistry (CHEM 1510 and CHEM 1520); LibreTexts, 2023. https://chem.libretexts.org/Courses/Thompson_Rivers_University/TRU%3A_Fundamentals_and_Principles_of_Chemistry_(CHEM_1510_and_CHEM_1520)/01%3A_Background/1.04%3A_Stoichiometry_of_Chemical_Reactions/1.4.02%3A_Writing_and_Balancing_Chemical_Equations.
4. Thompson Rivers University. TRU: Fundamentals and Principles of Chemistry (CHEM 1510 and CHEM 1520); LibreTexts, 2024. https://chem.libretexts.org/Courses/Thompson_Rivers_University/TRU%3A_Fundamentals_and_Principles_of_Chemistry_(CHEM_1510_and_CHEM_1520).
5. Thompson Rivers University. 3.E: Thermochemistry (Exercises). In TRU: Fundamentals and Principles of Chemistry (CHEM 1510 and CHEM 1520); LibreTexts, 2022. https://chem.libretexts.org/Courses/Thompson_Rivers_University/TRU%3A_Fundamentals_and_Principles_of_Chemistry_(CHEM_1510_and_CHEM_1520)/03%3A_Thermochemistry/3.E%3A_Thermochemistry_(Exercises).
6. Anonymous. Chapter 9.4: Heats of Formation. In General Chemistry: An Atoms First Approach; LibreTexts, 2025. https://chem.libretexts.org/Courses/Howard_University/General_Chemistry%3A_An_Atoms_First_Approach/Unit_4%3A__Thermochemistry/09%3A_Thermochemistry/Chapter_9.04%3A__Heats_of_Formation.