Organic Chemistry — Bonding and Structure: Identify Hybridization and Sketch Orbital Overlap
You may choose to prevent this website from aggregating and analyzing the actions you take here. Doing so will protect your privacy, but will also prevent the owner from learning from your actions and creating a better experience for you and other users.
This opt out feature requires JavaScript.
Question
For the molecule acetonitrile:
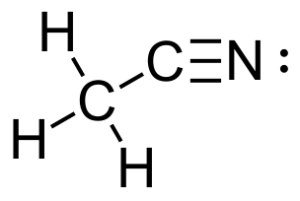
- How many sigma and pi bonds does it have?
- What orbitals overlap to form the C-H sigma bonds?
- What orbitals overlap to form the C-C sigma bonds?
- What orbitals overlap to form the C-N sigma bonds?
- What orbitals overlap to form the C-N pi bonds?
- What orbital contains the lone pair electrons on nitrogen?
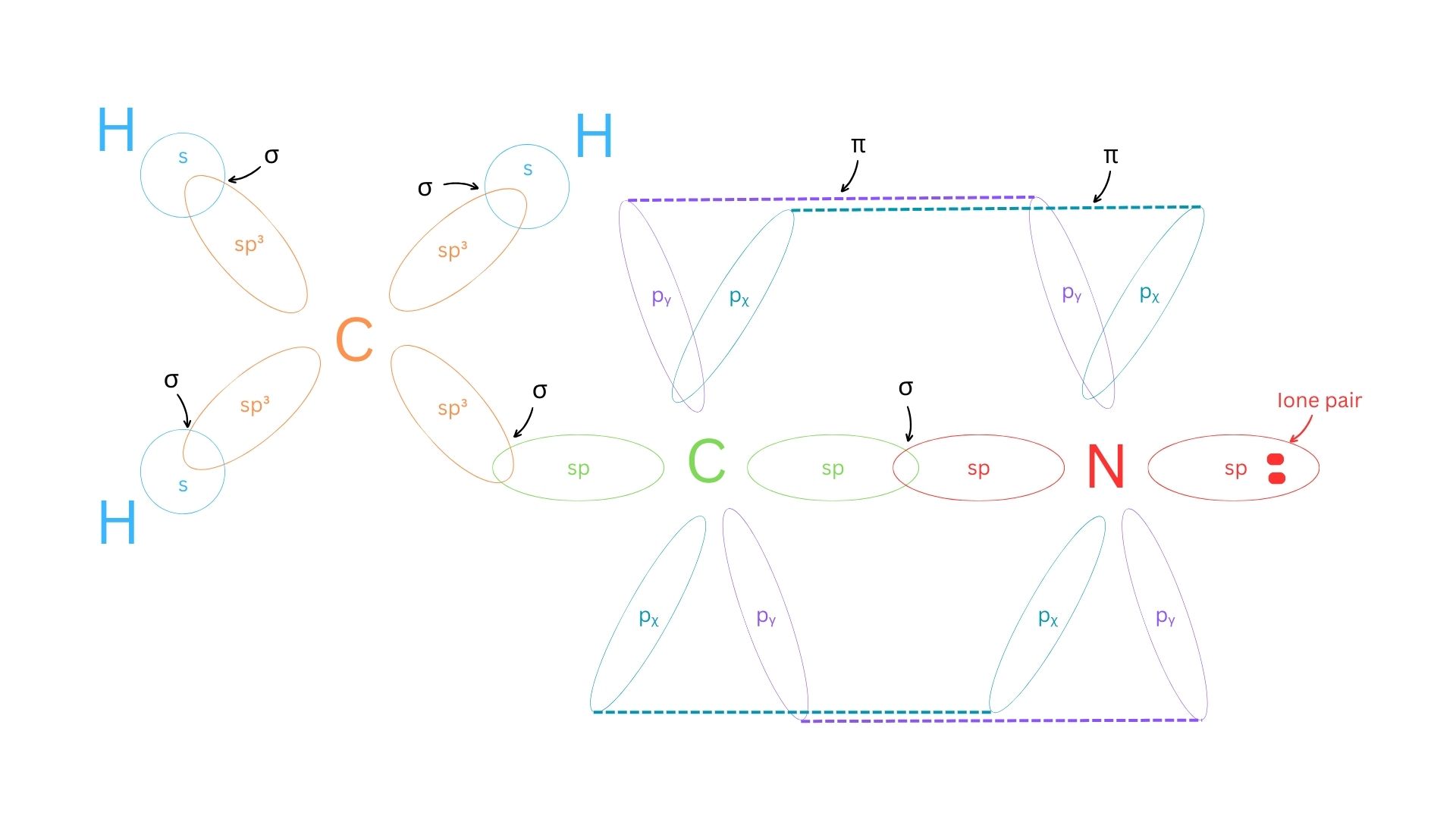
- Sketch the molecule showing the hybridized atomic orbital ‘cloud’ overlap.
Show/Hide Answer
- 5 sigma and 2 pi.
- A sp3 hybrid orbital from carbon and an s orbital from hydrogen.
- A sp3 hybrid orbital from one carbon and a sp hybrid orbital from another carbon.
- A sp hybrid orbital from carbon and a sp orbital from nitrogen.
- A py and a pz orbital from carbon and a py and pz orbital from nitrogen.
- A sp hybrid orbital.
- Sketch below:
Refer to Section 7.1.7: sp Hybrid Orbitals and the Structure of Acetylene (1).
Strategy Map
Do you need a little help to get started?
Check out the strategy map.
Show/Hide Strategy Map
| Strategy Map Steps |
|---|
1. Count the number of single bonds (sigma) the molecule has.
Show/Hide HintThere is a single bond between every single atom. |
2. Count the number of double and triple bonds (pi) the molecule has.
Show/Hide HintDouble and triple bonds are represented as second and third lines along a single bond. |
3. Identify the hybridization of center atoms (the two carbon atoms).
Show/Hide HintThe hybridization of the center atoms will depend on their electron domain geometry. |
| 4. Identify the hybridization of the outer atoms, if any. |
| 5. Identify which atoms are overlapping. The hybrid orbitals that overlap will depend on steps 3 and 4. |
| 6. Sketch your diagram showing all overlaps and ensure you label everything. |
Solution
Do you want to see the steps to reach the answer?
Check out this solution.
Show/Hide Solution
a. How many sigma and pi bonds does it have?
Answer: 5 sigma (single bonds) and 2 pi (1 double and 1 triple bond).
b. What orbitals overlap to form the C-H sigma bonds?
The hydrogen atoms are all unhybridized.
Answer: A sp3 hybrid orbital from carbon and an s orbital from hydrogen
c. What orbitals overlap to form the C-C sigma bonds?
Both carbons are hybridized, but their electron domain geometries are not the same, meaning their hybridizations are different.
Answer: A sp3 hybrid orbital from one carbon and a sp hybrid orbital from another carbon
d. What orbitals overlap to form the C-N sigma bonds?
Both carbon and nitrogen have a sp hybridization.
Answer: A sp hybrid orbital from carbon and a sp orbital from nitrogen
e. What orbitals overlap to form the C-N pi bonds?
The ‘Y’ and ‘Z’ mean they have slightly different orientations.
Answer: A py and a pz orbital from carbon and a py and pz orbital from nitrogen
f. What orbital contains the lone pair electrons on nitrogen?
Nitrogen has a linear electron domain geometry, meaning it has a sp hybridization in both directions.
Answer: A sp hybrid orbital
g. Sketch the molecule showing the hybridized atomic orbital ‘cloud’ overlap.
Answer:

Guided Solution
Do you want more help?
The guided solution below will give you the reasoning for each step to get your answer, with reminders and hints.
Show/Hide Guided Solution
| Guided Solution Ideas |
|---|
This question is a theory type problem that tests your knowledge on atomic orbital hybridization. You must correctly identify the hybridization of multiple atoms in a molecule and sketch the orbital overlaps.
Show/Hide Resource
|
| What are sigma and pi bonds?
Recall that:
Show/Hide Don’t Forget!There is at least 1 sigma bond between each atom. Look at the bonds between your carbon and your nitrogen. How many lines do you see? |
How do you identify atomic orbital hybridization?
Show/Hide Think About This!For instance, a tetrahedral must make 4 bonds, so it has an s + p + p + p (sp3) hybridization.
|
How do you know which orbitals are overlapping?
Show/Hide Think About This!Each bond that is between the atoms will be an overlap. The hybridization of the orbitals will depend on the 2 atoms the bond is between. |
How do you sketch the orbital overlaps?
|
| Complete Solution |
|---|
| a. How many sigma and pi bonds does it have?
There will always be a single bond between each atom in the molecule. A second or third line next to the single bond represents a double or triple bond. Count these in your Lewis structure form to identify the sigma and pi bonds. Answer: 5 sigma (single bonds) and 2 pi (1 double and 1 triple bond). |
| b. What orbitals overlap to form the C-H sigma bonds?
This carbon atom makes bonds in four directions, which gives it a sp3 hybridization. Three of these directions are hydrogen atoms. All of these hydrogens are unhybridized and have s orbitals. When the orbitals from the hydrogens and carbon overlap, they form a sigma bond. Answer: A sp3 hybrid orbital from carbon and an s orbital from hydrogen |
| c. What orbitals overlap to form the C-C sigma bonds?
Although both atoms are hybridized, they have a different number of bonding directions, and therefore, one is sp3 and the other is sp. Answer: A sp3 hybrid orbital from one carbon and a sp hybrid orbital from another carbon |
| d. What orbitals overlap to form the C-N sigma bonds?
Both atoms bond in two different directions giving them sp orbital hybridization. Answer: A sp hybrid orbital from carbon and a sp orbital from nitrogen |
| e. What orbitals overlap to form the C-N pi bonds?
These are the pi bonds. They are labelled with ‘Y’ and ‘Z’ as they have slightly different orientations on the axis. Pi orbitals make bonds above and below the sigma bond as they overlap sideways. Answer: A py and a pz orbital from carbon and a py and pz orbital from nitrogen. |
| f. What orbital contains the lone pair electrons on nitrogen?
Nitrogen has two electron domain directions. One is towards the carbon in a triple bond, and the other is towards its lone pair; therefore, it has two hybridized sp orbitals. The orbital that contains nitrogen’s lone pair is an sp orbital. Answer: A sp hybrid orbital. |
| g. Sketch the molecule showing the hybridized atomic orbital ‘cloud’ overlap.
Answer:
|
Check Your Work
Review your answers with the sketch and the structure to make sure you have tracked all the bonds and orbitals correctly. There are sigma bonds between each of the connected atoms, and the two pi bonds are the second and third bond between the C and N. Make sure that your identified orbitals match the electron domain geometry.
Does your answer make chemical sense?
Show/Hide Check Your Work!
Hybridized orbitals are created so all single bonds are made by the same orbitals and are equal. This is why hybridization will depend on the number of bonding directions the atom makes. The hybridization will likely vary between the atoms in the bond but will remain consistent within an atom itself.
Provide feedback by taking the survey here: link to survey

PASS Attribution
- LibreTexts PASS Chemistry Book CHEM 1500 (2).
- Question 7.1.7 from LibreTexts PASS Chemistry Book CHEM 1500 (3) is used under a CC BY-NC-SA 4.0 license.
- Question 7.1.7 is adapted from question 1 from LibreTexts CHEM 1500: Chemical Bonding and Organic Chemistry (1) is used under a CC BY-SA 4.0 license.
- Question 1 is question 1 from LibreTexts Organic Chemistry (Morsch et al.) (4), which is under a CC BY-SA 4.0 license.
Media Attributions
- Figure 1: Acetonitrile by Farmer et al. from LibreTexts CHEM 1500: Chemical Bonding and Organic Chemistry (1) is modified and used under a CC BY-SA 4.0 license.
- Figure 2: Hybridized atomic orbital ‘cloud’ overlap by the authors (Brewer S. and Blackstock L.), is free to use under a CC0 license.
References
1. Farmer, S.; Kennepohl, D.; Cunningham, K.; Soderburg, T.; Reusch, W. 7.1.7: sp Hybrid Orbitals and the Structure of Acetylene. In CHEM 1500: Chemical Bonding and Organic Chemistry; LibreTexts, 2023. https://chem.libretexts.org/Courses/Thompson_Rivers_University/CHEM1500%3A_Chemical_Bonding_and_Organic_Chemistry/07%3A_Organic_Chemistry_I_-_Bonding_and_Structure/7.01%3A_Bonding_and_Structure_I-_Review_of_Bonding/7.1.07%3A_sp_Hybrid_Orbitals_and_the_Structure_of_Acetylene.
2. Blackstock, L.; Brewer, S.; Jensen, A. PASS Chemistry Book CHEM 1500; LibreTexts, 2023. https://chem.libretexts.org/Courses/Thompson_Rivers_University/PASS_Chemistry_Book_CHEM_1500.
3. Blackstock, L.; Brewer, S.; Jensen, A. 7.1: Question 7.1.7 PASS – Label Hybridized Atomic Orbitals, Identify Sigma and Pi Bonds. In PASS Chemistry Book CHEM 1500; LibreTexts, 2023. https://chem.libretexts.org/Courses/Thompson_Rivers_University/PASS_Chemistry_Book_CHEM_1500/07%3A_Organic_Chemistry_I_-_Bonding_and_Structure/7.01%3A_Question_7.1.7_PASS_-_label_hybridized_atomic_orbitals_identify_sigma_and_pi_bonds.
4. Farmer, S.; Kennepohl, D.; Cunningham, K.; Soderburg, T.; Reusch, W. 1.9: sp Hybrid Orbitals and the Structure of Acetylene. In Organic Chemistry (Morsch et al.); LibreTexts, 2023. https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Organic_Chemistry_(Morsch_et_al.)/01%3A_Structure_and_Bonding/1.09%3A_sp_Hybrid_Orbitals_and_the_Structure_of_Acetylene.

