Organic Chemistry — Bonding and Structure: Interpret Chemical Structure Line Drawing
You may choose to prevent this website from aggregating and analyzing the actions you take here. Doing so will protect your privacy, but will also prevent the owner from learning from your actions and creating a better experience for you and other users.
This opt out feature requires JavaScript.
Question
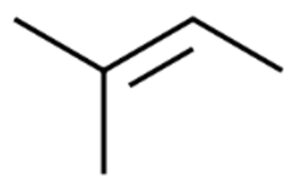
How many carbons are in the following drawing? How many hydrogens?
Show/Hide Answer
5 carbons; 10 hydrogens
Refer to Section 7.2: Drawing Chemical Structures (1).
Strategy Map
Do you need a little help to get started?
Check out the strategy map.
Show/Hide Strategy Map
| Strategy Map Steps |
|---|
1. Identify the carbons in the line drawing for the molecule.
Show/Hide HintIn a line structure, carbon atoms are found at the end of each line as well as at each corner. |
2. Add hydrogen atoms to the carbons until each carbon has 4 bonds.
Show/Hide HintThis will satisfy the octet rule. |
| 3. Count the carbon atoms and the hydrogen atoms in the molecule. |
Solution
Do you want to see the steps to reach the answer?
Check out this solution.
Show/Hide Solution
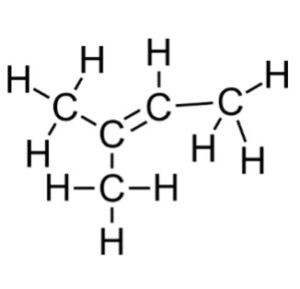 The full Lewis Structure shows that this molecule has 5 carbon atoms and 10 hydrogen atoms.
The full Lewis Structure shows that this molecule has 5 carbon atoms and 10 hydrogen atoms.
Guided Solution
Do you want more help?
The guided solution below will give you the reasoning for each step to get your answer, with reminders and hints.
Show/Hide Guided Solution
| Guided Solution Ideas |
|---|
This question is a theory problem that requires you to identify all implied carbons and hydrogens in a line structure.
Show/Hide Resource
|
What do the lines represent? Where are the implied carbons and hydrogens?
Show/Hide Don’t Forget!Recall that in a line structure, carbon atoms are found at the end of each line as well as at each corner. Hydrogen atoms surround the carbons to satisfy the octet rule. |
Draw the structure of the molecule showing all carbon and hydrogen atoms. Remember to fulfill the octet rule.
Show/Hide Don’t Forget!Recall your steps for drawing Lewis Structures. Refer to Section 4.4: Lewis Symbols and Structures (2). |
| Complete Solution |
|---|
| In a line structure, carbons are at either end of each line as well as in every corner. A double line shows the double bond.
Start by writing in the carbon atoms and their connectivity based on the line diagram. Show/Hide Structure!
|
Then, add hydrogen atoms to each C atom until each C has 4 bonds.
Show/Hide Structure!
|
| Count the carbon and hydrogen atoms shown in the structure.
Answer: In this molecule, there are 5 carbon atoms and 10 hydrogen atoms. |
Check Your Work
Summary of what we would expect based on the related chemistry theory
Show/Hide Check Your Work!
Counting valence electrons, there are 4 for each C and 1 for each H, making a total of 30 valence electrons. The Lewis Structure shows 30 valence electrons and satisfies the octet rule.
Does your answer make chemical sense?
Show/Hide Answer
The full structure represents the Lewis Structure for this molecule. There are 5 carbon atoms, which matches the line structure given in the question, and 10 hydrogen atoms, which satisfies the octet rule for each carbon.
There are many ways to draw molecules. A line structure gives us the ability to quickly create carbon-hydrogen structures. By convention, we know that each line represents the carbon-to-carbon bond and that all hydrogen atoms are “hidden.”
Refer to Section 4.4: Lewis Symbols and Structures (2).
Provide feedback by taking the survey here: link to survey

PASS Attribution
- LibreTexts PASS Chemistry Book CHEM 1500 (3).
- Question 7.2 from LibreTexts PASS Chemistry Book CHEM 1500 (4) is used under a CC BY-NC-SA 4.0 license.
- Question 7.2 is question 7.2.2 from LibreTexts CHEM 1500: Chemical Bonding and Organic Chemistry (1) is used under a CC BY-SA 4.0 license.
- Question 7.2.2 is question 1.12.2 from LibreTexts Organic Chemistry (Morsch et al.) (5), which is under a CC BY-SA 4.0 license.
Media Attributions
- All figures are by Ashlynn Jensen, from LibreTexts PASS Chemistry Book CHEM 1500 (4) by Blackstock et al., and are used/updated under a CC BY-NC 4.0 license.
References
1. Farmer, S.; Kennepohl, D.; Cunningham, K.; Soderburg, T.; Reusch, W. 7.2: Drawing Chemical Structures. In CHEM 1500: Chemical Bonding and Organic Chemistry; LibreTexts, 2022. https://chem.libretexts.org/Courses/Thompson_Rivers_University/CHEM_1500%3A_Chemical_Bonding_and_Organic_Chemistry/07%3A_Organic_Chemistry_I_-_Bonding_and_Structure/7.02%3A_Drawing_Chemical_Structures.
2. Elles, L. S. 4.4 Lewis Symbol and Structures. In CHEM 130 and 135: General Chemistry I and II (Sharpe Elles); LibreTexts, 2023. https://chem.libretexts.org/Courses/University_of_Kansas/CHEM_130%3A_General_Chemistry_I_(Sharpe_Elles)/04%3A_Chemical_Bonding_and_Molecular_Geometry/4.04%3A_Lewis_Symbols_and_Structures#:~:text=We%20use%20Lewis%20symbols%20to,period%20of%20the%20periodic%20table.
3. Blackstock, L.; Brewer, S.; Jensen, A. PASS Chemistry Book CHEM 1500; LibreTexts, 2023. https://chem.libretexts.org/Courses/Thompson_Rivers_University/PASS_Chemistry_Book_CHEM_1500.
4. Blackstock, L.; Brewer, S.; Jensen, A. 7.2: Question 7.2 PASS – Interpret Chemical Structure Line Drawing. In PASS Chemistry Book CHEM 1500; LibreTexts, 2023. https://chem.libretexts.org/Courses/Thompson_Rivers_University/PASS_Chemistry_Book_CHEM_1500/07%3A_Organic_Chemistry_I_-_Bonding_and_Structure/7.02%3A_Question_7.2_PASS_-_interpret_chemical_structure_line_drawing.
5. Farmer, S.; Kennepohl, D.; Cunningham, K.; Soderburg, T.; Reusch, W. 1.12: Drawing Chemical Structures . In Organic Chemistry (Morsch et al.); LibreTexts, 2023. https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Organic_Chemistry_(Morsch_et_al.)/01%3A_Structure_and_Bonding/1.12%3A_Drawing_Chemical_Structures.