Organic Chemistry — Conformational Analysis: Butane Newman Projection, Bond Rotation Energy Diagram
You may choose to prevent this website from aggregating and analyzing the actions you take here. Doing so will protect your privacy, but will also prevent the owner from learning from your actions and creating a better experience for you and other users.
This opt out feature requires JavaScript.
Question
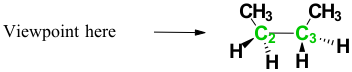
Consider butane’s C2—C3 bond:
- Draw the Newman projection as shown above.
- Draw the energy profile when the back carbon (C3) is rotated in 60° clockwise intervals.
- Label each conformation.
Show/Hide Answer
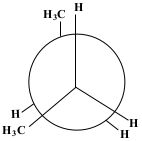
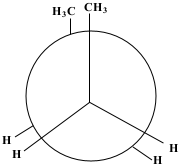
a. Newman Projection:
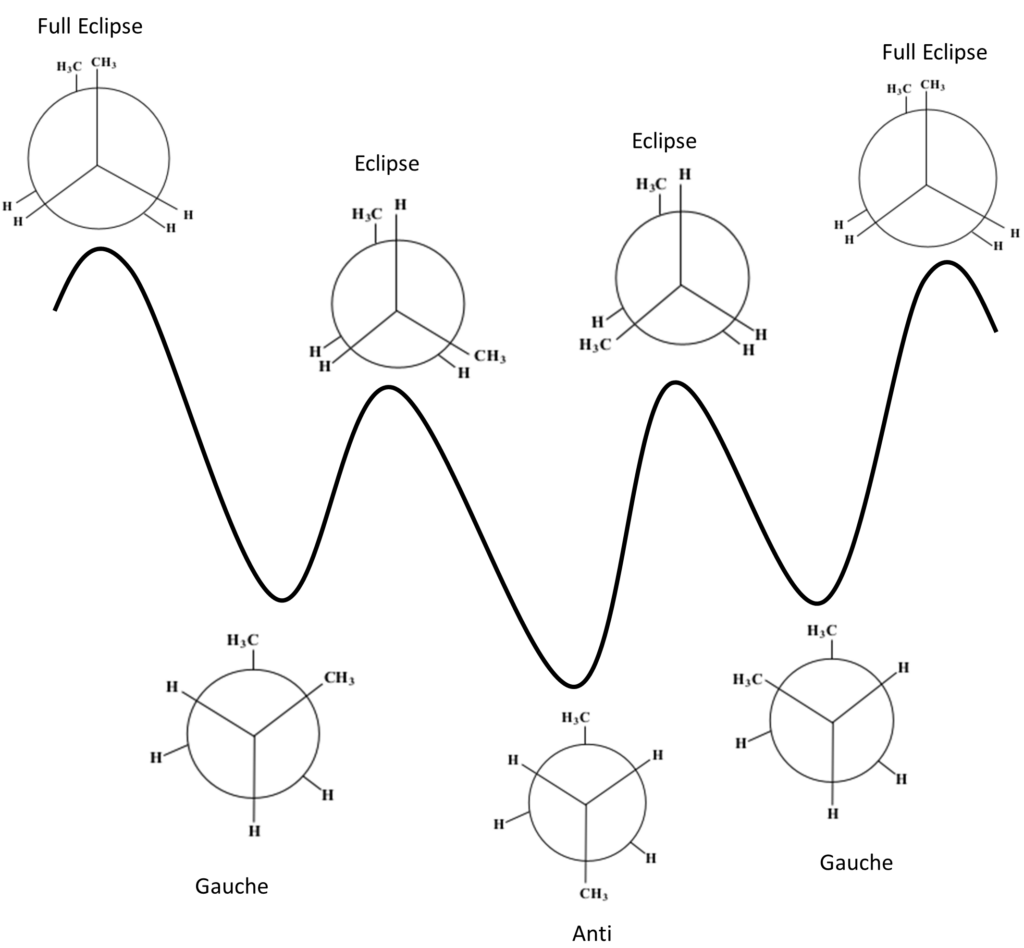
b. & c. Energy profile with labelled conformations:
Refer to Section 9.1: Rotation About Single Bonds- Conformations (1).
Strategy Map
Do you need a little help to get started?
Check out the strategy map.
Show/Hide Strategy Map
| Strategy Map Steps |
|---|
| 1. Using the viewpoint given in the question, sketch the Newman projection of the compound. |
| 2. Sketch each phase as the back carbon (C3) rotates clockwise. |
3. Create a graph depicting the energy change caused by the stability of each position.
Show/Hide HintThe closer the atoms are, the more interactions there will be. The further they are, the more stable the compound. |
4. Label each position with its corresponding name.
Show/Hide Watch Out!The possible names include Staggered Gauche, Eclipse, Staggered Anti, and Full Eclipse. These are not in any particular order. |
Solution
Do you want to see the steps to reach the answer?
Check out this solution.
Show/Hide Solution
a. Newman Projection:
 The exterior circle represents the forward carbon (C2) and the interior centre represents the back carbon (C3).
The exterior circle represents the forward carbon (C2) and the interior centre represents the back carbon (C3).
b. & c. Energy profile with labelled conformations:
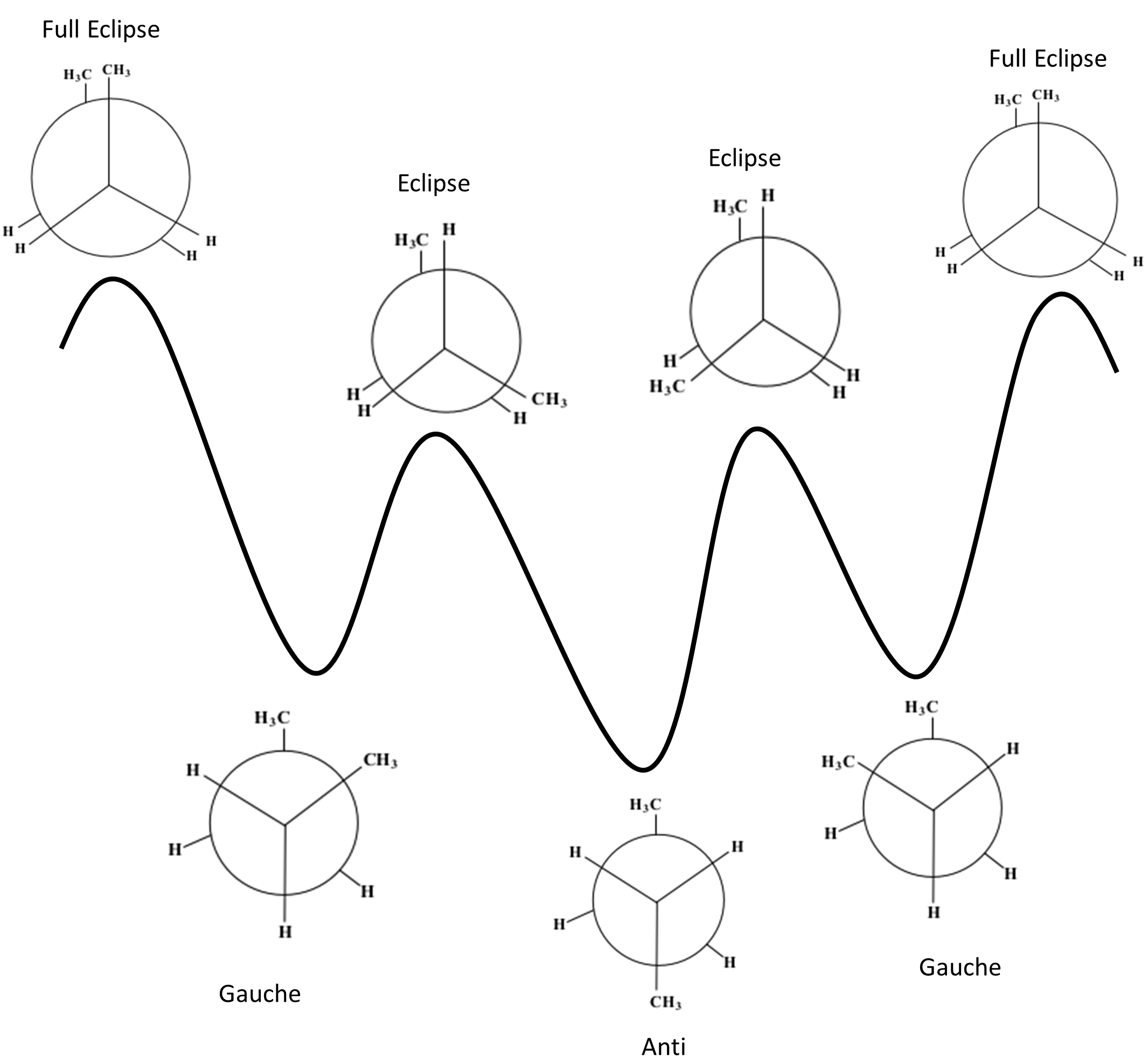
The diagram shows the 7 stages of a butane compounds rotation as well as the different levels of stability for each.
From least stable to most stable: Full Eclipse < Eclipse < Staggered Gauche < Staggered Anti
Guided Solution
Do you want more help?
The guided solution below will give you the reasoning for each step to get your answer, with reminders and hints.
Show/Hide Guided Solution
| Guided Solution Ideas |
|---|
This question is a theory type problem that tests your knowledge on the different conformations of a compound and the stability of each conformation.
Show/Hide Resource
|
Identify your viewpoint (which bond are you looking at).
Show/Hide Think About This!
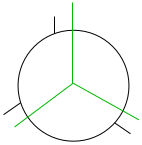
Looking down the carbon-carbon bond, C2 is in the front and C3 is in the back. Draw the skeleton shape of your compound. A line represents the back carbon, and a circle represents the front carbon. Show/Hide Think About This!
In the above figure, the back carbon is represented in green, and the front carbon is represented in black. Draw the groups attached to the front carbon. Show/Hide Think About This!
The figure above is drawn with the substituent groups on the front carbon. Draw the attached groups to the back carbon. Show/Hide Think About This!
The figure above is drawn with the substituent groups on the back carbon. |
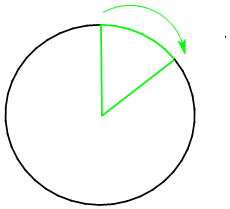
| How much is a 60° rotation?
There are 360° in a circle. If the bond rotates 60°, it is rotating 1/6 of a circle. Show/Hide Think About This!
|
Show/Hide Think About This!Due to interactions between different atoms, certain positions will have a higher stability than others. Certain positions will cause more atoms to be in closer contact, and they must overcome more repulsions. This requires energy and, therefore, causes the compound to be less stable. |
| Recall the name of each conformation.
The possible names include Staggered Gauche, Eclipse, Staggered Anti and Full Eclipse. These are not in any particular order. Show/Hide Don’t Forget!Full Eclipse:
Staggered Gauche:
Staggered Anti:
Eclipse:
|
| Complete Solution |
|---|
| Newman Projection:
The figure above depicts the compound butane down the carbon-carbon bond. The exterior circle represents the forward carbon (C2) and the interior centre represents the back carbon (C3). The back carbon will rotate at 60° angles. Energy profile with labeled conformations:
The above diagram shows the 7 stages of a butane compounds rotation as well as the different levels of stability for each. From least to most stable: Full Eclipse < Eclipse < Staggered Gauche < Staggered Anti |
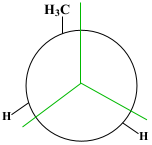
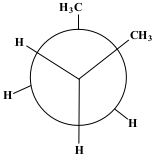
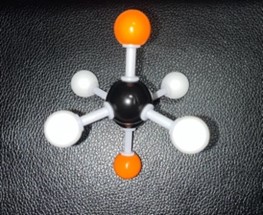
The starting phase is Full Eclipse, with both of the methane groups side by side. This position requires the most energy and, therefore, is the least stable position.
Show/Hide Think About This!Molecular model of the Newman projection in Full Eclipse:
|
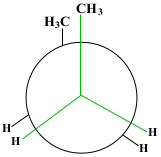
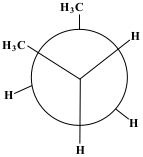
After the first 60° rotation, the position of the compound is called Staggered Gauche. The methane group on the back carbon (C3) is now to the left of the methane group on the front carbon (C2). This position is significantly more stable.
Show/Hide Think About This!Molecular model of the Newman projection in Staggered Gauche:
|
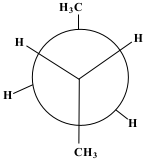
After the next 60° rotation, the position of the compound is called Eclipse. The methane on the back carbon (C3) is now overlapping with the hydrogen on the front carbon (C2). This position is slightly less stable than the last.
Show/Hide Think About This!
|
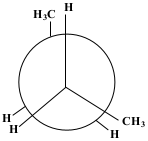
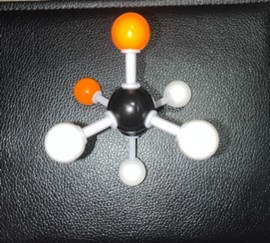
After the next 60° rotation, the position of the compound is called Staggered Anti. The methane group on the back carbon (C3) is now in the opposite position to the methane group on the front carbon (C2). This position is the most stable.
Show/Hide Think About This!Molecular model of the Newman projection in Staggered Anti:
|
After the next 60° rotation, the position of the compound is called Eclipse. The methane on the back carbon (C3) is now overlapping with the hydrogen on the front carbon (C2). This position is less stable than the last.
Show/Hide Think About This!Molecular model of the Newman projection in Eclipse:
|
After the next 60° rotation, the position of the compound is called Staggered Gauche. The methane group on the back carbon (C3) is now to the right of the methane group on the front carbon (C2). This position is more stable than the previous last.
Show/Hide Think About This!Molecular model of the Newman projection in Staggered Gauche:
|
| After one more 60° rotation, the compound is back to the first position of Full Eclipse. |
Check Your Work
Molecules are constantly moving around in a 3D space. Single bonds will twist and rotate. Due to interactions between different atoms, certain positions will have higher stability than others. We can analyze the stability of these positions by looking at the atoms in close contact.
Show/Hide Check Your Work!
Ensure that your viewpoint perspective remains constant throughout the duration of solving the problem. If the question defines a specific viewpoint, be sure to use it or your answer may be reversed.
Does your answer make chemical sense?
Show/Hide Answer
Developing and reviewing your answer makes most logical sense when you work through each 60o rotation systematically in clockwise order (rotating the back carbon). Unless specifically instructed, start in a conformation that is either most or least stable (i.e., largest groups furthest apart or largest groups closest together, respectively).
Recall the figure with energy profile and labelled conformations in the solution section above to visualize the different relative stabilities. Starting in the least stable, highest energy conformation called full eclipse (i.e., with the two largest groups, methyls, in closest proximity to each other), working through each rotation to staggered and then eclipsed, the energy states will fluctuate until we return back to full eclipse.
Provide feedback by taking the survey here: link to survey

PASS Attribution
- LibreTexts PASS Chemistry Book CHEM 1500 (3).
- Question 9.E.1 from LibreTexts PASS Chemistry Book CHEM 1500 (4) is used under a CC BY-NC-SA 4.0 license.
Media Attributions
- All drawn figures by Ashlynn Jensen and Esther Ojukwu, from LibreTexts PASS Chemistry Book CHEM 1500 (4), are used under a CC BY-NC 4.0 license.
- All photo figures by Ashlynn Jensen, from LibreTexts PASS Chemistry Book CHEM 1500 (4), are used under a CC BY-NC 4.0 license.
References
1. LibreTexts. 9.1: Rotation about Single Bonds- Conformations. In CHEM 1500: Chemical Bonding and Organic Chemistry; LibreTexts, 2023. https://chem.libretexts.org/Courses/Thompson_Rivers_University/CHEM1500%3A_Chemical_Bonding_and_Organic_Chemistry/09%3A_Organic_Chemistry_III_-_Conformational_Analysis/9.04%3A_Cyclohexane-_A_Strain-Free_Cycloalkane#How_to_Draw_the_Chair_Conformation.
2. Blackstock, L; Brewer, S.; Jensen, A. 9.1: Question 9.E.1 PASS – butane Newman projection, bond rotation energy diagram. In PASS Chemistry Book CHEM 1500; LibreTexts, 2023. https://chem.libretexts.org/Courses/Thompson_Rivers_University/PASS_Chemistry_Book_CHEM_1500/09%3A_Organic_Chemistry_III_-_Conformational_Analysis/9.03%3A_Question_9.E.2new_PASS_-_cycloalkane_chair_axial_vs._equatorial_most_stable.