Organic Chemistry – Stereochemistry: Alkenes, Label E or Z
You may choose to prevent this website from aggregating and analyzing the actions you take here. Doing so will protect your privacy, but will also prevent the owner from learning from your actions and creating a better experience for you and other users.
This opt out feature requires JavaScript.
Question
Label the following alkenes as E, Z, or neither:
-
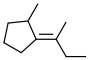
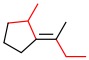
Alkene A -
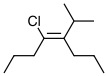
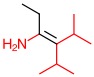
Alkene B, Alkenes, Label E or Z 1 -
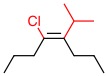
Alkene C
Show/Hide Answer
- E
- Z
- Neither
Refer to Section 8.5.2 Sequence Rules – The E,Z Designation (1).
Strategy Map
Do you need a little help to get started?
Check out the strategy map.
Show/Hide Strategy Map
| Strategy Map Steps |
|---|
1. Look to the left side of the double bond. Using the laws for priority, identify if the substituent with the higher priority is on the top or bottom half of the double bond.
Show/Hide HintBoth substituents may have the same priority. |
| 2. Repeat step 1 with the right side. |
| 3. Identify if the higher priority substituents are both on one side or opposite sides of the double bond. |
Solution
Do you want to see the steps to reach the answer?
Check out this solution.
Show/Hide Solution
a. Alkene A
The substituents with higher priorities are on opposite sides.
Answer: E
b. Alkene B
The substituents with higher priorities are on the same side.
Answer: Z
c. Alkene C
Both substituents on the right have the same priority.
Answer: Neither
Guided Solution
Do you want more help?
The guided solution below will give you the reasoning for each step to get your answer, with reminders and hints.
Show/Hide Guided Solution
| Guided Solution Ideas |
|---|
This question is a theory type problem where you must identify the geometry of alkene structures by labeling them as either E, Z, or neither.
Show/Hide Resource
|
| First, determine the 2 substituents on each double-bonded carbon separately.
Rank these substituents based on the atom directly attached to the double-bonded carbon. The substituent whose atom has a higher atomic number takes precedence over the substituent whose atom has a lower atomic number. If the atoms are the same, look to the next atom they are bonded to. (From Section 8.5.2 Sequence Rules – The E,Z Designation (1)) |
How do you assign substituent priority?
Show/Hide Resource!Refer to Section 8.4: Absolute Configuration- R-S Sequence Rules (2). |
What does it mean for an alkene to have a type E geometry?
Show/Hide Don’t Forget!If it has a type E geometry, the highest priority substituents are on opposite sides of the double bond (one is on the top and the other on the bottom). |
What does it mean for an alkene to have a type Z geometry?
Show/Hide Don’t Forget!If it has a type Z geometry, the highest priority substituents are on the same side of the double bond (both on the top or bottom). |
What does it mean if an alkene has neither?
Show/Hide Don’t Forget!If it has neither of these, it means that the substituents on one side (left or right) have the same priority. |
| Complete Solution |
|---|
| a. Alkene 1
The priority substituents are highlighted in red, and the higher priorities are on opposite sides of the double bond.
Answer: E |
| b. Alkene 2
The priority substituents are highlighted in red, and the higher priorities are on the same side of the double bond.
Answer: Z |
| c. Alkene 3
Both of the substituents showing on the right side of the double bond above have the same priority, so it is neither E or Z.
Answer: Neither |
Check Your Work
Summary of what we would expect based on the related chemistry theory
Show/Hide Check Your Work!
Go back and review your priority assignments for double bond substituents. Make sure you go atom by atom when looking for priority.
Does your answer make chemical sense?
Show/Hide Answer
The naming rules are used in order to fully describe these molecules when other naming systems do not work. Your answer should make sense if you are following all guidelines to identify priority and geometry.
Provide feedback by taking the survey here: link to survey

PASS Attribution
- LibreTexts PASS Chemistry Book CHEM 1500 (3)
- Question 8.5.2.3 from LibreTexts PASS Chemistry Book CHEM 1500 (4) is adapted under a CC BY-NC 4.0 license.
- Questions 8.5.2.3 is adapted from question 7.6.3 from LibreTexts Organic Chemistry (Morsch et al.) (5), which is under a CC BY-SA 4.0 license.
Media Attributions
All figures are by Farmer et al., from Organic Chemistry (Morsch et al.) (5), and are used under a CC BY-SA 4.0 license.
References
1. Thompson Rivers University. 8.5.2: Sequence Rules – The E,Z Designation. In CHEM 1500: Chemical Bonding and Organic Chemistry; LibreTexts, 2023. https://chem.libretexts.org/Courses/Thompson_Rivers_University/CHEM_1500%3A_Chemical_Bonding_and_Organic_Chemistry/08%3A_Organic_Chemistry_II_-_Stereochemistry/8.05%3A_Stereochemistry_of_the_Alkenes/8.5.02%3A_Sequence_Rules_-_The_EZ_Designation.
2. Thompson Rivers University. 8.4: Absolute Configuration- R-S Sequence Rules. In CHEM 1500: Chemical Bonding and Organic Chemistry; LibreTexts, 2022. https://chem.libretexts.org/Courses/Thompson_Rivers_University/CHEM_1500%3A_Chemical_Bonding_and_Organic_Chemistry/08%3A_Organic_Chemistry_II_-_Stereochemistry/8.04%3A_Absolute_Configuration-_R-S__Sequence_Rules.
3. Blackstock, L.; Brewer, S.; Jensen, A. PASS Chemistry Book CHEM 1500; LibreTexts, 2023. https://chem.libretexts.org/Courses/Thompson_Rivers_University/PASS_Chemistry_Book_CHEM_1500.
4. Blackstock, L.; Brewer, S.; Jensen, A. 8.1. Question 8.5.2.3 PASS – Alkenes, Label E or Z. In PASS Chemistry Book CHEM 1500; LibreTexts, 2023. https://chem.libretexts.org/Courses/Thompson_Rivers_University/PASS_Chemistry_Book_CHEM_1500/08%3A_Organic_Chemistry_II_-_Stereochemistry/8.01%3A_Question_8.5.2.3_PASS_-_alkenes_label_E_or_Z.
5. Farmer, S.; Kennepohl, D.; Sharrett, Z.; Cunningham, K.; Roberts, J.; Caserio, M. C.; Bruner, R. 7.6: Sequence Rules – The E,Z Designation. In Organic Chemistry (Morsch et al.); LibreTexts, 2024. https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Organic_Chemistry_(Morsch_et_al.)/07%3A_Alkenes-_Structure_and_Reactivity/7.06%3A_Sequence_Rules_-_The_EZ_Designation.