Quantum Theory and Electronic Structure: Quantum Numbers, Orbital Characteristics
You may choose to prevent this website from aggregating and analyzing the actions you take here. Doing so will protect your privacy, but will also prevent the owner from learning from your actions and creating a better experience for you and other users.
This opt out feature requires JavaScript.
Question
Consider the orbitals shown below…
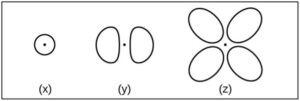
- What is the maximum number of electrons contained in an orbital of type (x)? Of type (y)? Of type (z)?
- How many orbitals of type (x) are found in a shell with n = 2? How many of type (y)? How many of type (z)?
- Write a set of quantum numbers for an electron in an orbital of type (x) in a shell with n = 4. Of an orbital of type (y) in a shell with n = 2. Of an orbital of type (z) in a shell with n = 3.
- What is the smallest possible n value for an orbital of type (x)? Of type (y)? Of type (z)?
- What are the possible l and ml values for an orbital of type (x)? Of type (y)? Of type (z)?
Show/Hide Answer
- Type (x) = 2 electrons
Type (y) = 2 electrons
Type (z) = 2 electrons - Type (x) = 1 orbital
Type (y) = 3 orbitals
Type (z) = 0, does not exist - Type (x) = [4, 0, 0, ½]
Type (y) = [2, 1, 0, -½]
Type (z) = [3, 2, -1, ½] - Type (x): n = 1
Type (y): n = 2
Type (z): n = 3 - Type (x): l =0, ml =0
Type (y): l = 1, ml = 1, 0, -1
Type (z): l = 2, ml = 2, 1, 0, -1, -2
Refer to:
- Figure 2.5.7 in Section 2.4: Quantum Mechanics and the Atom (1) for a visual representation of the orbital types and their suborbital positions.
- Figure 2.6.3 in Section 2.6, Electronic Structure of Atoms (Electron Configurations) (2) for a visual representation of the hierarchy of subshell filling/energy levels.
Strategy Map
Do you need a little help to get started?
Check out the strategy map.
Show/Hide Strategy Map
| Strategy Map Steps |
|---|
1. Identify what orbital types are represented in the figure.
Show/Hide HintIn general chemistry, 4 types of orbitals are introduced: s, p, d, and f. Recall this mnemonic, where the letter can help describe the shape:
|
2. Consider each requirement of this multi-part problem and work through the answer step by step to not miss anything.
Show/Hide HintBe familiar with the fundamental concepts of atomic orbital theory and the key characteristics of each orbital type. Consider: Hund’s Rule, Aufbau’s Principle, and the Pauli Exclusion Principle to guide your thinking. |
Solution
Do you want to see the steps to reach the answer?
Check out this solution.
Show/Hide Solution
All Types
a. What is the maximum number of electrons contained in an orbital of type (x)? Of type (y)? Of type (z)?
Answer: Any orbital can house a total of 2 electrons (1 with spin up + ½ and 1 with spin down – ½).
Type (x)
The shape of this orbital is spherical (s-type; 1 lobe; no planar nodes).
s-type orbitals have the following characteristics:
- n is equal to or greater than 1.
- l is always equal to 0 (defines the shape).
- ml is always equal to 0 (only 1 distinct orientation).
- there is only ever 1 s-type orbital per n-level
- ms can be equal to -½ or +½
Answers:
- 1 orbital
- [4, 0, 0, ½]
- n = 1
- l = 0, ml = 0
Type (y)
The shape of this orbital is a dumbbell or propellor (p-type; 2 lobes; 1 planar node in x-, y-, or z-axis).
p-type orbitals have the following characteristics:
- n is equal to or greater than 2; p-type orbitals cannot exist when n = 1.
- l is always equal to 1 (defines the shape).
- ml can be -1, 0, or 1 (3 distinct orbital orientations).
- there can be up to 3 p-type orbitals per n-level (when n is greater than or equal to 2).
- ms can be equal to -½ or +½
Answers:
- 3 orbitals
- [2, 1, 0, -½]
- n = 2
- l = 1; ml = -1, 0, 1
Type (z)
The shape of this orbital is a clover or daisy (d-type; typically 4 lobes; typically 2 planar nodes).
d-type orbitals have the following characteristics:
- n is equal to or greater than 3; d-type orbitals cannot exist when n = 1 or 2.
- l is always equal to 2 (defines the shape).
- ml can be -2, -1, 0, 1, or 2 (5 distinct orbital arrangements)
- there can be up to 5 d-type orbitals per n-level (when n is greater than or equal to 3).
- ms can be equal to -½ or +½.
Answers:
- 0 orbitals, does not exist
- [3, 2, -1, ½]
- n = 3
- l = 2, ml = 2, 1, 0, -1, -2
Guided Solution
Do you want more help?
The guided solution below will give you the reasoning for each step to get your answer, with reminders and hints.
Show/Hide Guided Solution
| Guided Solution Ideas |
|---|
This problem requires comprehension of the following concepts:
Consider the orbitals shown below:
Show/Hide Resource
|
Recall the quantum numbers.
Show/Hide Don’t Forget!Quantum numbers: [n, l, ml, ms] What are the quantum numbers, and what do they represent? What are the guidelines/rules/principles that inform each quantum number? Show/Hide Don’t Forget!
|
| Complete Solution |
|---|
| a. What is the maximum number of electrons contained in an orbital of type (x)? Of type (y)? Of type (z)?
Think about how many electrons can you put into any orbital. Think about electron pairing. Regardless of orbital type, each orbital can contain at most 2 electrons; 1 electron spins in a direction (ms = +½), and the other electron spins in the opposite direction (ms=-½). Answer:
|
| b. How many orbitals of type (x) are found in a shell with n = 2? How many of type (y)? How many of type (z)?
First, identify each orbital type based on the shape in the provided figure. Here, we must consider what quantum numbers are allowed for each orbital type when n = 2.
Consider the relationship between quantum numbers n and l when n = 2; is each orbital type (i.e., x, y, and z) allowed? Recall: l must be 0 or a positive whole number integer; restricted by n (i.e., l = 0, 1, 2, … n-1).
Summary: A Type (z) or ‘d-type’ orbital does not exist in the n = 2 shell. Answer:
|
| c. Write a set of quantum numbers for an electron in an orbital of type (x) in a shell with n = 4. Of an orbital of type (y) in a shell with n = 2. Of an orbital of type (z) in a shell with n = 3.
Consider what each quantum number represents. Summarize the quantum numbers you have been provided in the question. Recall there are 4 distinct quantum numbers [n, l, ml, ms]. The question provides the principal quantum number (n), and the shape of the orbital defines the angular momentum quantum number (l):
What quantum numbers must be defined to answer the question? Are there any restrictions that must be considered? Still need ml and ms:
Is there more than one correct answer? Yes, there is more than one allowed quantum number set for each orbital type, as defined in the question. Type (x) = [n = 4, l = 0, ml = 0, ms = +½]
Type (y) = [n = 2, l = 1, ml = 0, ms = -½]
Type (z) = [n = 3, l = 2, ml = -1, ms = +½]
Answer:
|
| d. What is the smallest possible n value for an orbital of type (x)? Of type (y)? Of type (z)?
Consider the relationship between quantum numbers n and l. Recall: l must be 0 or a positive whole number integer; the value of l is restricted by n (i.e., l = 0, 1, 2, … n-1; l≠n). Therefore, the lowest value of n = (l + 1). Recall the value of l for each type of orbital:
Using those l values, you can calculate the lowest n for each type:
Answer:
|
| e. What are the possible l and ml values for an orbital of type (x)? Of type (y)? Of type (z)?
Consider the relationship between ml and l quantum numbers. ml must be a positive or negative whole number integer, or zero; ml is restricted by l (i.e., ml = -l, … -2, -1, 0, 1, 2, … +l). Recall the value of l for each type of orbital:
Using those l values, you can figure out the possible ml values for each type:
Answer:
|
Check Your Work
Summary of what we would expect based on the related chemistry theory.
Show/Hide Check Your Work!
Review the atomic orbital shapes and their characteristics (s-, p-, and d-orbitals) as well as which combinations of quantum numbers are and are not allowed to exist.
Recall the related fundamental principles that guide atomic orbital theory:
- Pauli Exclusion Principle — no two electrons can have the same 4 quantum numbers.
- Aufbau’s Principle — electrons occupy the lowest energy orbital available first.
Does your answer make chemical sense?
Show/Hide Answer
For question b, our answers on the number of orbitals in a sublevel make sense because:
- An s-orbital (sphere-shaped electron density cloud) is an identical sphere regardless of its orientation in space, resulting in only 1 possible s-orbital at each energy level.
- A p-orbital (propellor-shaped electron density cloud) can exist in3 different axes in 3-dimensional space; it can lay along the x-axis, y-axis, and z-axis, resulting in 3 possible p-orbitals (px, py, and pz) at each energy level.
Provide feedback by taking the survey here: link to survey

PASS Attribution
-
- LibreTexts PASS Chemistry Book CHEM 1500 (4).
- Question 2.E.40 from LibreTexts PASS Chemistry Book CHEM 1500 (5) is used under a CC BY-NC-SA 4.0 license.
- Question 2.E.40 was adapted question 6.3.11 from LibreTexts Chemistry 1e (OpenSTAX) (6), which is under a CC BY 4.0 license.
- Question 6.1.6 is question 41 from OpenStax Chemistry 2e (7), which is under a CC BY 4.0 license. Access for free at https://openstax.org/books/chemistry-2e/pages/1-introduction.
Media Attributions
All figures by the authors (Brewer S. and Blackstock L.) are free to use under a CC0 license.
References
1. Thompson Rivers University. 2.4: Quantum Mechanics and The Atom. In CHEM 1500: Chemical Bonding and Organic Chemistry. LibreTexts, 2023. https://chem.libretexts.org/Courses/Thompson_Rivers_University/CHEM1500%3A_Chemical_Bonding_and_Organic_Chemistry/02%3A_Quantum_Theory_and_Electronic_Structure_of_Atoms/2.02%3A_Atomic_Spectroscopy_and_The_Bohr_Model.
2. OpenStax. 2.6: Electronic Structure of Atoms (Electron Configurations). In CHEM 1500: Chemical Bonding and Organic Chemistry. LibreTexts, 2023. https://chem.libretexts.org/Courses/Thompson_Rivers_University/CHEM_1500%3A_Chemical_Bonding_and_Organic_Chemistry/02%3A_Quantum_Theory_and_Electronic_Structure_of_Atoms/2.06%3A_Electronic_Structure_of_Atoms_(Electron_Configurations)
3. Thompson Rivers University. 2.5: The Shape of Atomic Orbitals. In CHEM 1500: Chemical Bonding and Organic Chemistry. LibreTexts, 2022. https://chem.libretexts.org/Courses/Thompson_Rivers_University/CHEM_1500%3A_Chemical_Bonding_and_Organic_Chemistry/02%3A_Quantum_Theory_and_Electronic_Structure_of_Atoms/2.05%3A_The_Shape_of_Atomic_Orbitals
4. Blackstock, L.; Brewer, S.; Jensen, A. PASS Chemistry Book CHEM 1500; LibreTexts, 2023. https://chem.libretexts.org/Courses/Thompson_Rivers_University/PASS_Chemistry_Book_CHEM_1500.
5. Blackstock, L.; Brewer, S.; Jensen, A. 2.3: Question 2.E.40 PASS – Quantum Numbers, Orbital Characteristics. In PASS Chemistry Book CHEM 1500. LibreTexts, 2023. https://chem.libretexts.org/Courses/Thompson_Rivers_University/PASS_Chemistry_Book_CHEM_1500/02%3A_Quantum_Theory_and_Electronic_Structure/2.03%3A_Question_2.E.40_PASS_-_quantum_numbers_orbital_characteristics.
6. OpenStax. 6.E: Electronic Structure and Periodic Properties (Exercises). In Chemistry 1e (OpenSTAX). LibreTexts, 2023. https://chem.libretexts.org/Bookshelves/General_Chemistry/Chemistry_1e_(OpenSTAX)/06%3A_Electronic_Structure_and_Periodic_Properties/6.E%3A_Electronic_Structure_and_Periodic_Properties_(Exercises)
7. Flowers, P.; Robinson, W. R.; Langley, R.; Theopold, K. Ch. 6 Exercises. In Chemistry 2e; OpenStax, 2019. https://openstax.org/books/chemistry-2e/pages/6-exercises