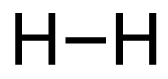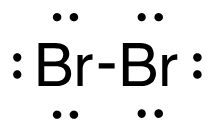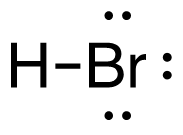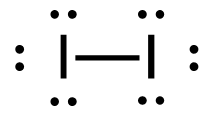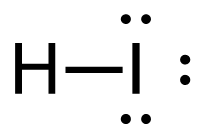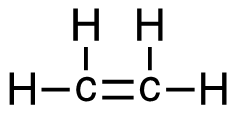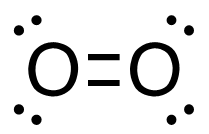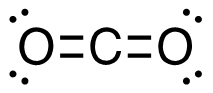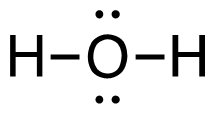Chemical Bonding — Basic Concepts: Calculate Enthalpy Change from Bond Energy
You may choose to prevent this website from aggregating and analyzing the actions you take here. Doing so will protect your privacy, but will also prevent the owner from learning from your actions and creating a better experience for you and other users.
This opt out feature requires JavaScript.
Question
Using the bond energies in Table 4.6.1: Bond Energies (kJ/mol) (1), determine the approximate enthalpy change for each of the following reactions:
- H2(g) + Br2(g) → 2HBr(g)
- CH4(g) + I2(g) → CH3I(g) + HI(g)
- C2H4(g) + 3O2 → 2CO2(g) + 2H2O(g)
Show/Hide Answer
- −114 kJ
- 30. kJ
- −1465 kJ (use 799 kJ/mol for C=O bond energy in CO2)
Refer to Section 4.6: Strengths of Ionic and Covalent Bonds (1).
Strategy Map
Do you need a little help to get started?
Check out the strategy map.
Show/Hide Strategy Map
| Strategy Map Steps |
|---|
1. Identify which molecules have bonds broken and which have bonds formed.
Show/Hide HintReactant bonds break, and product bonds form in a chemical reaction. |
2. Create Lewis structures for each of the molecules to know what bonds there are.
Show/Hide HintIf you are unsure which elements are bonded to which, try making a Lewis Structure of the molecule. Refer to Section 4.4 Lewis Symbols and Structures (2). |
3. Use the provided table to look up the corresponding bond energies.
Show/Hide HintTable 4.6.1: Bond Energies (kJ/mol) from Section 4.6: Strengths of Ionic and Covalent Bonds (1). |
4. Plug the values into the enthalpy change equation and solve for each reaction.
Show/Hide HintBreaking bonds requires an energy input. When bonds form, energy is released. When energy is released, it is negative. Recall the equation that demonstrates this relationship. |
Solution
Check out this solution.
Show/Hide Solution
a. H2 (g) + Br2 (g) → 2HBr (g)
[latex]\begin{align} \Delta& \mathrm{H}=\left(\sum \text { Bonds broken }\right)-\left(\sum \text { Bonds formed }\right) \\\\ \Delta& \mathrm{H}=(1(\mathrm{H-H})+1(\mathrm{Br-Br}))-(2(\mathrm{H-Br})) \\\\ \Delta& \mathrm{H}=(1 \mathrm{~mol}(436\mathrm{~kJ}/ \mathrm{mol})+1\mathrm{~mol}(190. \mathrm{~kJ}/\mathrm{mol}))-(2 \mathrm{~mol}(370. \mathrm{~kJ}/ \mathrm{mol})) \\\\ \Delta& \mathrm{H}=(626 \mathrm{~kJ})-(740. \mathrm{~kJ}) \\\\ \Delta& \mathrm{H}=-114 \mathrm{~kJ} \end{align}[/latex]
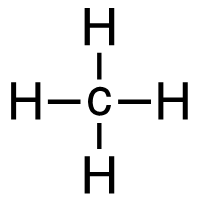
b. CH4 (g) + I2 (g) → CH3I (g) + HI (g)
[latex]\begin{align} \Delta& \mathrm{H}=\left(\sum \text { Bonds broken }\right)-\left(\sum \text { Bonds formed }\right) \\\\ \Delta& \mathrm{H=(4(C-H)+(I-I))-(3(C-H)+(C-I)+(H-I))} \\\\ \Delta& \mathrm{H=(4~mol(415 ~kJ/mol)+1~mol(150. ~kJ/mol))-(3~mol(415 ~kJ/mol)\\+1~mol(240. ~kJ/mol)+1~mol(295 ~kJ/mol))} \\\\ \Delta& \mathrm{H=(1660~kJ+150. ~kJ)-(1245 ~kJ + 240. ~kJ + 295 ~kJ)} \\\\ \Delta& \mathrm{H=(1810 ~kJ)-(1780 ~kJ)} \\\\ \Delta& \mathrm{H}=\mathrm{30.} \mathrm{~ kJ} \end{align}[/latex]
c. C2H4 (g) + 3O2 (g)→ 2CO2 (g) + 2H2O (g)
[latex]\begin{align} \Delta& \mathrm{H}=\left(\sum \text { Bonds broken }\right)-\left(\sum \text { Bonds formed }\right) \\\\ \Delta& \mathrm{H=(4(C-H)+(C=C)+3(O=O))-(2(2(C=O))+2(2(H-O)))} \\\\ \Delta& \mathrm{H=(4 ~mol(415 ~kJ/mol)+1 ~mol(611 ~kJ/mol)+3~mol(498 ~kJ/mol))\\-(4 mol(741 ~kJ/mol)+4 ~mol(464 ~kJ/mol))} \\\\ \Delta& \mathrm{H=((1660 ~kJ + 611 ~kJ) + (1494 ~kJ - 2964 ~kJ + (1856 ~kJ)} \\\\ \Delta& \mathrm{H=(3765 ~kJ)-(4820 ~kJ)} \\\\ \Delta& \mathrm{H}=-\mathrm{1 0 5 5} \mathrm{~kJ} = 1.05_5\mathrm{x}10^3\mathrm{~kJ} \end{align}[/latex]
Guided Solution
The guided solution below will give you the reasoning for each step to get your answer, with reminders and hints.
Show/Hide Guided Solution
| Guided Solution Ideas |
|---|
This question is a calculation problem in which you must identify what bonds are broken and formed during a chemical reaction. This type of problem requires you to use a table of values to calculate the overall energy change in the reaction using bond energies.
Show/Hide Resource
|
It can be difficult to know how many bonds are in a molecule. The easiest way to find what bonds there are is to create a Lewis Structure of the molecule.
Show/Hide Resource
|
Recall that bonds require energy to break (start a reaction), and energy is released when bonds form (in the products of a reaction).
Show/Hide Don’t Forget![latex]\Delta H=\left(\sum \text {Bonds broken}\right)-\left(\sum \text {Bonds formed}\right)[/latex] The energy of bonds formed is subtracted as they are formed in the reaction. Bond energy values, by definition, are the energy required to break the bond. By subtracting the sum of the product bonds formed, we are changing the sign of the energy to represent the opposite process (forming the bond). |
When using the bond energies table, scan the table for your desired bond (i.e. (H-H)). The number beside it will be the one used in the calculation. Make sure you do not confuse single, double, and triple bonds!
Show/Hide Don’t Forget!The bond energy for H-H is 436 kJ. |
| Complete Solution |
|---|
| The following equation will be used for each of the reactions:
[latex]\Delta H=\left(\sum \text { Bonds broken }\right)-\left(\sum \text { Bonds formed }\right)[/latex] For a reaction to occur, bonds need to break, and new bonds need to form. When bonds break, energy is absorbed. When bonds form, energy is released. We use this to calculate the sum of the energy change over the reaction. How do we know which bonds are breaking and which are forming? Show/Hide Don’t Forget!In a reaction, the bonds to the left of the arrow will break, and the bonds to the right will form. ___(break)___ → ___(form)___ |
| a. H2 (g) + Br2 (g) → 2HBr (g)
[latex]\Delta H=\left(\sum \text { Bonds broken }\right)-\left(\sum \text { Bonds formed }\right)[/latex] The bond between the 2 hydrogen and the 2 bromine atoms breaks. Two product molecules form, each with a hydrogen bonded to a bromine. Show/Hide Structures
[latex]\Delta \mathrm{H}=(1 \operatorname{mol}\mathrm{(H-H)+1} \operatorname{mol}\mathrm{(B r-B r))-(2} \operatorname{mol}\mathrm{(H-B r))}[/latex] Using Table 4.6.1: Bond Energies (kJ/mol) from Section 4.6: Strengths of Ionic and Covalent Bonds (1), the values for each bond energy replaces their respective bonds in the equation. [latex]\Delta \mathrm{H=(1} \mathrm{~mol}(436 \mathrm{~kJ} / \mathrm{mol})+1 \mathrm{~mol}(190 . \mathrm{kJ} / \mathrm{mol}))-(2 \mathrm{~mol}(370 . \mathrm{kJ} / \mathrm{mol}))[/latex] When solving inside the brackets, keep stoichiometry in mind. [latex]\Delta \mathrm{H=(626 k J)-(740 . k J)}[/latex] The energy released from the bonds formed is subtracted from the energy absorbed by the bonds broken. ΔH=-114 kJ The calculated value is negative, meaning the reaction was exothermic. |
| b. CH4 (g) + I2 (g) → CH3I (g) + HI (g)
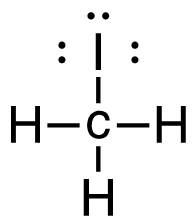
[latex]\Delta \mathrm{H}=\left(\sum \text { Bonds broken }\right)-\left(\sum \text { Bonds formed }\right)[/latex] Four carbon-hydrogen and 1 iodine-iodine bonds break. Three carbon-hydrogen bonds, 1 carbon-iodine, and 1 hydrogen-iodine are formed. Show/Hide Think About This!Make sure you have the correct bonds for CH4 and CH3I. Show/Hide Structures
[latex]\Delta \mathrm{H=(4(C-H)+(I-I))-(3(C-H)+(C-I)+(H-I))}[/latex] Using Table 4.6.1: Bond Energies (kJ/mol) from Section 4.6: Strengths of Ionic and Covalent Bonds (1), the values for each bond energy replaces their respective bonds in the equation. [latex]\begin{align} \Delta& \mathrm{H=(4mol(415 kJ/mol)+1 mol(150. kJ/mol))-(3 mol(415 kJ/mol)\\+1 mol(240. kJ/mol)+1 mol(295 kJ/mol))} \\\\ \Delta& \mathrm{H=((1660 kJ)+(150. kJ))-((1245 kJ)+(240. kJ)+(295 kJ))} \end{align}[/latex] When solving inside the brackets, keep stoichiometry in mind. [latex]\Delta \mathrm{H=(1810 kJ)-(1780 kJ)}[/latex] The energy released from the bonds formed is subtracted from the energy absorbed by the bonds broken. ΔH=30 kJ The calculated value is positive, meaning the reaction was endothermic |
| c. C2H4 (g) + 3O2 (g) → 2CO2 (g) + 2H2O (g)
[latex]\Delta \mathrm{H}=\left(\sum \text { Bonds broken }\right)-\left(\sum \text { Bonds formed }\right)[/latex] Two hydrogen-carbon, 1 carbon-carbon double, and 3 oxygen-oxygen double bonds break. Two molecules of CO2 form that contain 2 carbon-oxygen double bonds and 2 molecules, each containing 2 hydrogen-oxygen bonds. Show/Hide Think About This!Make sure you have the correct bonds for C2H4, O2 and CO2. Show/Hide Structures
[latex]\begin{gathered} \Delta \mathrm{H=(4(C-H)+(C=C)+3(O=O))-(2(2(C=O))+2(2(H-O)))} \end{gathered}[/latex] Using Table 4.6.1: Bond Energies (kJ/mol) from Section 4.6: Strengths of Ionic and Covalent Bonds (1), the values for each bond energy replaces their respective bonds in the equation. [latex]\begin{gathered} \Delta \mathrm{H=(4 mol(415 kJ/mol)+1 mol(611 kJ/mol)\\ +3 mol(498 kJ/mol))-(4 mol(741 kJ/mol)\\ +4 mol(464 kJ/mol))} \end{gathered}[/latex] When solving inside the brackets, keep stoichiometry in mind. [latex]\begin{align} \Delta& \mathrm{H=((1660 kJ)+(611 kJ)+(1494 kJ))-((2964 kJ)+(1856 kJ))} \\\\ \Delta& \mathrm{H=(3765 kJ)-(4820 kJ)} \end{align}[/latex] The energy released from the bonds formed is subtracted from the energy absorbed by the bonds broken. ΔH= -1055 kJ = 1.055 x 103 kJ The calculated value is negative, meaning the reaction was exothermic. |
Check Your Work
When comparing the bond energy values, we see that the product bonds (2 H-Br) have a greater magnitude than the reactant bonds (H-H and Br-Br), so we would expect this reaction to have an exothermic ΔH.
Show/Hide Check Your Work!
Make sure you add up all bonds and consider the reaction stoichiometry.
Does your answer make chemical sense?
Show/Hide Answer
When any reaction occurs, energy will be absorbed and released; however, the overall sum can be endothermic (requires more energy than it releases) or exothermic (releases more energy than it requires). We can numerically observe this using the enthalpy change from the bond energies equation.
Provide feedback by taking the survey here: link to survey

PASS Attribution
- LibreTexts PASS Chemistry Book CHEM 1500 (3).
- Question 4.E.65 in LibreTexts PASS Chemistry Book CHEM 1500 (4) is used under a CC BY-NC-SA 4.0 license.
- Question 4.E.65 is a question from Section 7.E.5: 7.6: Strengths of Ionic and Covalent Bonds from LibreTexts Chemistry 1e (OpenSTAX) (5), which is under a CC BY 4.0 license.
- The question from Section 7.E.5: 7.6: Strengths of Ionic and Covalent Bonds is question 66 from OpenStax Chemistry 2e (6), which is under a CC BY 4.0 license. Access for free at https://openstax.org/books/chemistry-2e/pages/1-introduction.
Media Attributions
All figures by the authors (Brewer S. and Blackstock L.) are free to use under a CC0 license.
References
1. OpenStax. 4.6: Strengths of Ionic and Covalent Bonds. In CHEM 1500: Chemical Bonding and Organic Chemistry; LibreTexts, 2022. https://chem.libretexts.org/Courses/Thompson_Rivers_University/CHEM1500%3A_Chemical_Bonding_and_Organic_Chemistry/04%3A_Chemical_Bonding_I-_Basic_Concepts/4.06%3A_Strengths_of_Ionic_and_Covalent_Bonds.
2. OpenStax. 4.4: Lewis Symbols and Structures. In CHEM 1500: Chemical Bonding and Organic Chemistry; LibreTexts, 2022. https://chem.libretexts.org/Courses/Thompson_Rivers_University/CHEM1500%3A_Chemical_Bonding_and_Organic_Chemistry/04%3A_Chemical_Bonding_I-_Basic_Concepts/4.04%3A_Lewis_Symbols_and_Structures.
3. Blackstock, L.; Brewer, S.; Jensen, A. PASS Chemistry Book CHEM 1500; LibreTexts, 2023. https://chem.libretexts.org/Courses/Thompson_Rivers_University/PASS_Chemistry_Book_CHEM_1500.
4. Blackstock, L.; Brewer, S.; Jensen, A. 4.3: Question 4.E.65 PASS – Determining Enthalpy Change From Bond Energies. In PASS Chemistry Book CHEM 1500; LibreTexts, 2023. https://chem.libretexts.org/Courses/Thompson_Rivers_University/PASS_Chemistry_Book_CHEM_1500/04%3A_Chemical_Bonding_I_-_Basic_Concepts/4.03%3A_Question_4.E.65_PASS_-_determining_enthalpy_change_from_bond_energies.
5. OpenStax. 7.E: Chemical Bonding and Molecular Geometry (Exercises). In Chemistry 1e (OpenSTAX); LibreTexts. 2022. https://chem.libretexts.org/Bookshelves/General_Chemistry/Chemistry_1e_(OpenSTAX)/07%3A_Chemical_Bonding_and_Molecular_Geometry/7.E%3A_Chemical_Bonding_and_Molecular_Geometry_(Exercises).
6. Flowers, P.; Theopold, K.; Langley, R.; Robinson, W. R. Ch. 7 Exercises. In Chemistry 2e; OpenStax, 2019. https://openstax.org/books/chemistry-2e/pages/7-exercises.