Organic Chemistry — Stereochemistry: Determine Stereocenter Configuration R or S
You may choose to prevent this website from aggregating and analyzing the actions you take here. Doing so will protect your privacy, but will also prevent the owner from learning from your actions and creating a better experience for you and other users.
This opt out feature requires JavaScript.
Question
For the following compounds, assign R or S configurations for each stereocenter.
-
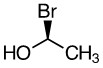
Compound A -
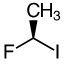
Compound B -
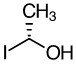
Compound C
Show/Hide Answer
- S
- R
- R
Refer to Section 8.4: Absolute Configuration- R-S Sequence Rules (1).
Strategy Map
Do you need a little help to get started?
Check out the strategy map.
Show/Hide Strategy Map
| Strategy Map Steps |
|---|
| 1. Identify chiral carbon with 4 different groups attached |
2. Assign priorities to substituents and label them 1 through 4.
Show/Hide Resource
|
| 3. Rotate the molecule so that the lowest priority group (#4) is facing away from you (towards the back) |
4. Count in the direction of 1 to 3 and identify if this is clockwise or counterclockwise.
Show/Hide HintClockwise = R |
Solution
Do you want to see the steps to reach the answer?
Check out this solution.
Show/Hide Solution
a. Compound A
- S
- Priority goes counterclockwise around the stereocenter.
b. Compound B
- R
- Priority goes clockwise around the stereocenter.
c. Compound C
- R
- The hydrogen (not shown in the line-dash-wedge structure) is wedged towards the viewer; therefore, the chirality will be the opposite.
- Priority goes counterclockwise (S) around the stereocenter as drawn; therefore, the chiral center is R.
Guided Solution
Do you want more help?
The guided solution below will give you the reasoning for each step to get your answer, with reminders and hints.
Show/Hide Guided Solution
| Guided Solution Ideas |
|---|
This question is a theory problem where you assign the correct configuration to a carbon stereocenter (R or S).
Show/Hide Resource
|
For the following compounds, assign R or S configurations for each stereocenter.
|
The rules to assign priority to substituents:
Show/Hide ResourceRefer to Section 8.4: Absolute Configuration- R-S Sequence Rules (1). |
| R vs S configuration
To identify the configuration of a stereocenter, you must identify if your order of priority goes in the clockwise or counterclockwise direction. Clockwise = R |
| Complete Solution |
|---|
a. Compound A
 Bromine is the first priority (atomic number 35). It goes counterclockwise around the stereocenter. Answer: S |
b. Compound B
 Iodine is the first priority (atomic number 53). It goes clockwise around the stereocenter. Answer: R |
c. Compound C
 Hydrogen is wedged forward, so you must rotate the molecule or reverse the stereochemistry determined, as drawn below. 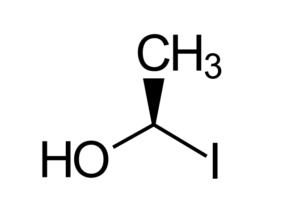 Hydrogen, the lowest priority group, is now dashed back. It goes counterclockwise around the stereocenter in the original figure. Answer: R |
Check Your Work
Check to make sure you are following the guidelines of labelling priority. Ensure you go atom by atom.
Does your answer make chemical sense?
Show/Hide Check Your Work!
We use the “right hand” and “left hand” nomenclature to name the enantiomers of a chiral compound. We label the stereocenters as (R) or (S).
These naming rules provide an unambiguous name that indicates the molecule’s stereochemistry.
Provide feedback by taking the survey here: link to survey

PASS Attribution
- LibreTexts PASS Chemistry Book CHEM 1500 (2).
- Questions 8.4.E.1 from LibreTexts PASS Chemistry Book CHEM 1500 (3) is used under a CC BY-NC 4.0 license.
Media Attributions
- Compound A by Blackstock et al. from LibreTexts PASS Chemistry Book CHEM 1500 (2) is used under a CC BY-NC 4.0 license.
- Compound B by Blackstock et al. from LibreTexts PASS Chemistry Book CHEM 1500 (2) is used under a CC BY-NC 4.0 license.
- Compound C by Blackstock et al. from LibreTexts PASS Chemistry Book CHEM 1500 (2) is used under a CC BY-NC 4.0 license.
- Rotated Compound C by Blackstock et al. from LibreTexts PASS Chemistry Book CHEM 1500 (2) is used under a CC BY-NC 4.0 license.
References
1. LibreTexts. 8.4: Absolute Configuration- R-S Sequence Rules. In CHEM1500: Chemical Bonding and Organic Chemistry; LibreTexts, 2022. https://chem.libretexts.org/Courses/Thompson_Rivers_University/CHEM1500%3A_Chemical_Bonding_and_Organic_Chemistry/08%3A_Organic_Chemistry_II_-_Stereochemistry/8.04%3A_Absolute_Configuration-_R-S__Sequence_Rules.
2. Blackstock, L.; Brewer, S.; Jensen, A. 8.2. PASS Chemistry Book CHEM 1500; LibreTexts, 2023. https://chem.libretexts.org/Courses/Thompson_Rivers_University/PASS_Chemistry_Book_CHEM_1500.
3. Blackstock, L.; Brewer, S.; Jensen, A. 8.2. Question 8.4.E.1 PASS – Stereocenter Configuration R or S. In PASS Chemistry Book CHEM 1500; LibreTexts, 2024. https://chem.libretexts.org/Courses/Thompson_Rivers_University/PASS_Chemistry_Book_CHEM_1500/08%3A_Organic_Chemistry_II_-_Stereochemistry/8.2._Question_8.4.E.1_PASS_-_stereocenter_configuration_R_or_S.

